Part I:the QCM as a mass sensor
In this experiment you will use the QCM as a mass sensor during deposition and dissolution of copper on a gold-covered QCM electrode.
How to run a single experiment:
- Set the current sign to (-) for deposition or (+) for dissolution.
- set the current knob to the desired value:
- Set the minimum and maximum Δf to the desired value. This will dictate the thickness of the layer that will be deposited or dissolved.
- Make sure that the "Manual STOP" button is green.
- Press the "Run" (arrow) button on the upper left.
- Press "Continue" to start the experiment.
- The process will stop automatically when the maximum Δf is reached.
| # | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| I (mA) | 0.3 | 1.49 | 3.56 | 7.99 |
Procedure:
- Assemble the setup.
- Pre-deposition of a safety layer: Deposit a layer with Δf = 20,000 Hz and I = 1.49 mA to guarantee that the gold electrode itself will not be dissolved.
- Run a deposition and dissolution experiment at each current. Set the frequency limits to ±5000 Hz. Make sure you save your results! Files are saved using the top panel: enter your group number (make sure a proper folder exists) and the file name, and turn turn the "Save" button to "ON".
- Run an experiment with the current set to 0. What is the process at work?
- Data analysis:
- Plot Δf vs. time for all the experiments on the same graph and explain it.
- Calculate Δm (use equation 5) and the coating thickness, w. Plot Δf as a function of w.
- Calculate Cm for each current using equation 2, and compare to the theoretical value.
- Plot the voltage as a function of time and as a function of current. What can you learn from the graph?
- Plot Δf vs. time for zero current. Explain your results.
The QCM as a viscometer
In this experiment you will use the QCM as a viscometer, and will measure the viscosity of several substances and mixtures.
- Measure the resonance frequency of methanol, ethanol and propanol:
- Record the frequency in air. This is f0.
- Pour some liquid into the teflon container.
- Wait for the reading to stabilize, and record the frequency and standard deviation.
- Cleaning the container: Turn over the container, and pour the liquid into a waste glass. Put on the lid, and fasten it with a rubber band. Let nitrogen gas flow in until the QCM electrode is completely dry, then turn the container right side up.
- When you insert liquids, don't drip directly on the electrode. Instead, let the liquid slowly drip on the sidewalls.
- When you pour out liquid, turn the container in one quick motion to make sure liquid doesn't drip on the sides. Use some paper to absorb any liquid that might have spilled on the sides.
- Avoid fluctuating the system!
- Prepare mixtures of ethanol and propanol with 4-5 different molar fractions. Measure their resonance frequencies according to steps a-d above.
- Repeat step 2 for mixtures of propanol and water.
- Data analysis:
- Calibration: plot Δf/ρ vs. (η/ρ)½ for the pure materials (use the known values of their density and viscosity, given in table 1), and find Cη according to equation 4.
- Calculate the molar fraction (use figure 9) and the density of your mixtures (for mixtures of alcohol and water, use figure 1. Alcohol mixtures have practically the same value).
- Use the density and calibration constants to calculate the viscosity of the mixture.
- Plot the viscosity as a function of the mole fraction. Explain the shape of the graph.
|
Pay attention! The QCM electrode is extremely sensitive. In order to ensure it will continue to function properly, follow these instructions: |
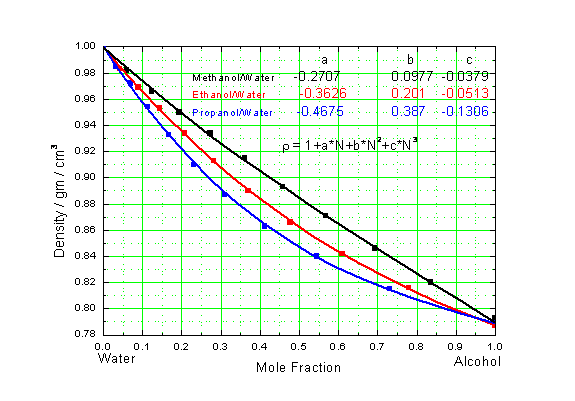 |
| Figure 1: Mole fractions of alcohol and water. |