Gas Adsorption
In this experiment you will learn about gas adsorption to a solid surface.
Preparation
Answer the following questions in writing:
- Can you measure the unknown volume differently from the method suggested in this laboratory experiment? How?
- Briefly explain the concepts of physical and chemical adsorption. Explain how the nature of the adsorption can be determined using the experimental results and our knowledge of the properties of adsorbed gases. Give three such indications.
- The BET isotherm of N2 gas on activated carbon was measured in an adsorption experiment at a temperature of 77K. The experimental results showed that c=5. For N2, ΔHvap is 2.7928 kJ/mol.
- What is the adsorption enthalpy?
- How will the isotherm change for different values of c?
- When will the behavior fit the Langmuir model better and when will it fit BET better?
- Which model better fits chemisorption and which fits physiosorption better?
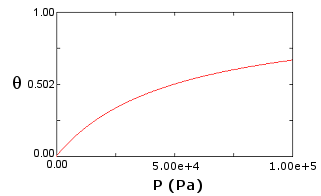
- Find the equilibrium constant K based on the graph below. Hint: Use Langmuir's model.
- Why do we have to heat the activated carbon before the experiment?
- What is the purpose of the trap?
- Why does the experiment have to be performed at liquid nitrogen temperature? How would the isotherms change if we performed the experiment at 60°C? At 100°C?
- What can we learn from the kinetics experiment? What can you say about the thermodynamics of the process? Refer to the changes in entropy, the enthalpy and the free energy.
- Do you expect the adsorption and desorption isotherms to be similar? Why?
Additionally, you should be familiar with the properties and operation principles of the pressure gauges and the rotary pump.