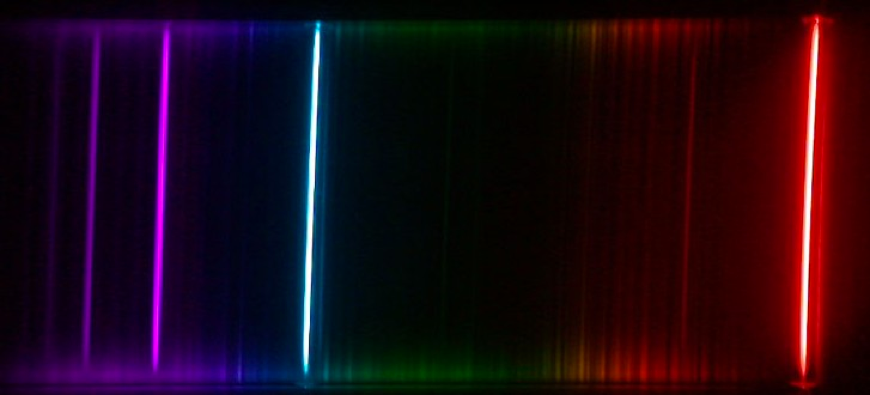
Spectrum of the hydrogen atom
Measure a part of the hydrogen atom spectrum and distinguish it from the spectrum of deuterium.
The hydrogen atom has only one orbital electron surrounding a nucleus consisting of a single proton; this is why it serves as a good model system to study energy levels of an atom. In this experiment you will apply spectroscopic techniques to study the hydrogen atom. Specifically you will:
- Obtain a spectrum of the Balmer series of the hydrogen atom and determine the Rydberg constant.
- Observe the isotopic shifts of hydrogen and deuterium and calculate the mass ratio of deuterium and proton.
- Learn the basic principles of spectroscopic experimental equipment.