Light Microscopy
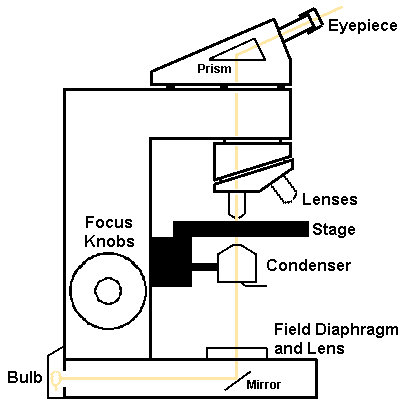
Light microscopy is the standard means for viewing tissues and cells and fluids on a glass slide. Light is focused from a light source through the condenser onto the glass slide which is positioned on the microscope stage. The stage contains controls that can move the slide, or it can be moved by hand. The light illuminates the slide and the image is captured via the passage of the light through a lens that rests just above the glass slide. The lens then focuses and magnifies the image, where it is viewed through the microscope's eyepiece(s).
A typical microscope has a series of lenses mounted on a rotating turret, so that the lenses can be changed and provide different levels of magnification. Standard lenses include magnifications of 4X, 10X, 40X, and 100X. Additional lenses can be added to provide different magnifications. Lenses that use air as the medium between the specimen and the lens are known as "dry" objectives while those (usually 50X or greater) that use an oil medium are known as "oil" objectives.
Lens quality can be rated according to the "numerical aperture" (N.A.). This is a measure of the ability of the lens to resolve, or distinguish, what is viewed, and not just magnify. The N.A. is calculated from multiplying the refractive index of the air (or oil) between which the light passes from the coverslip over the specimen to the lens and the sine of the angle that the outermost ray of light makes when entering the lens. A higher N.A. is better and is achieved by getting the lens closer to the specimen. In practice, a N.A. of 0.95 is the best that can be achieved with "dry" objectives, while a N.A. of 1.40 is the best with an "oil" objective.
The 2X and 4X lenses are considered "low power" or "scanning" lenses to view a wider area of the specimen. The 10X and 20X lenses provide a medium power for a closer view, while 40X to 60X lenses provide a high magnification. The 100X lens most often is made to be used with oil immersion, because that provides a better "numerical aperture" that improves image quality. Lower power objectives tend to have a lower N.A., which in practice is not critical, since these are designed to be "scanning" lenses for a wider view, unlike high power objectives that need better N.A. for the best resolution.
The quality of a lens (actually, a series of lenses positioned in a metal tube) is provided by certain aspects of its manufacture. Lenses tend to have spherical distortion (the image appears curved) and chromatic distortion (diffraction by the lens produces color halos in the image). Lenses can be manufactured to correct these aberrations, typically by combining a series of lens elements:
Aplanar lens: images appear flat across the field of view by correcting spherical distortion
Achromat lens: images are focused at the same point for different wavelengths to give less spherical disortion
Planachromat lens: combines aplanar and achromatic corrections to provide minimal spherical distortion
Apochromat lens: corrects for both spherical and chromatic aberrations
Planapochromat lens: corrects for both spherical and chromatic aberrations and gives a flat field of view
An achromat lens is the cheapest, but is quite usable for routine microscopy, while a "planapochromat" lens with the best qualities costs more, but provides a better image for critical photography.
The microscope has a "condenser" lens below the stage which focuses the light source on the specimen being observed. Condensers are adjustable to move up and down, to center the light source, and to cut the amount of light with a movable diaphragm. In addition, many microscopes have an adjustable lens (the field diaphragm) just above the light source (below the condenser) which helps to focus the light in the plane of the condenser. By proper adjustment of these elements, "Koehler" illumination can be obtained in which there is even illumination across the field of view.
Eyepieces can provide a narrow or wide field of view.
The light source, typically a halogen lamp bulb, provides a bright light that can be further filtered to adjust the color balance. Most light sources are controlled via a rheostat to adjust the intensity of the light. The usual halogen bulb provides light that is "tungsten" light of about 3200K in temperature, so that a camera used to capture the images from such a source must be loaded with appropriate "tungsten" type film, unless a compensating filter has been used. When using black and white film, a green filter will help to adjust the hues optimally.
Polarized Light Microscopy
Some materials have the property of "birefringence" which is the ability to pass light in a particular plane. Such materials are called "anisotropic" because of this property. These are typically crystals or fibers. Normally, most materials are "isotropic" because any light that passes through them will be scattered in all directions. When viewed under polarized light, however, anisotropic materials will be brightly visible in one plane ("birefringent"), but will be dark in a plane turned 90 degrees.
The birefringence observed with polarized light can be further subdivided into "positive" and "negative" birefrigence. This is based upon the property of birefringence in which rays of light travelling through the anisotropic material in perpendicular planes (at right angles) will travel at different velocities through the material. Thus, a birefringent material actually has two refractive indices, a higher one for the "fast" rays of light and smaller refractive index for the "slow" rays travelling through the material. These rays of light can also be called "ordinary" when they are reflected by the material and "extraordinary" when the rays pass straight through the material.
A substance is positively birefringent if the "ordinary" reflected ray becomes the "fast" ray that travels faster in parallel with the crystalline structure of the material than the "extraordinary" ray that is "slow" when it traverses the material. Negative birefringence occurs when the "ordinary" ray becomes the "slow" ray when it is reflected and travels across the crystalline structure.
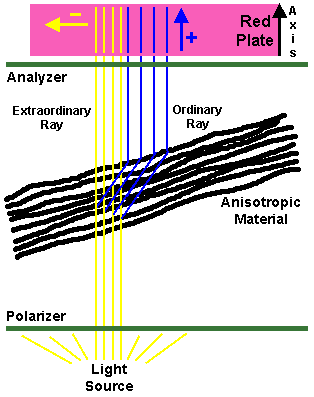
For polarized light microscopy, there must be two polarizing filters made of glass or plastic material which will pass light in only one plane. The material is generally made of a thickness that will absorb the "ordinary" or "slow" ray and pass the "fast" rays as "plane polarized" light. One filter (the "analyzer") is placed above the specimen (on top of the slide, or in a filter holder in the turret of the microscope above the lenses). The other (the "polarizer") is placed over the light source below the specimen on the glass slide. Polarizing sets made for a particular microscope can be purchased; however, a polarizing lens for a single lens reflex (SLR) camera can act as a good "polarizer" while an inexpensive sheet of plastic polarizing material can be cut to size to provide the "analyzer".
Properties of positive and negative birefringence can be assessed only with an additional "compensator" plate placed on top of the polarizer (or in the condenser). This compensator is made of a material (quartz or selenite) which magically retards the light a quarter wavelength and produces an interference pattern with a red background on which the properties of positive and negative birefringence can be seen. Thus, the compensator is often called a "red plate". A material is said to be positively birefringent when it appears blue if its axis is aligned parallel to the long axis of the compensator, or "red plate". A negatively birefringent material will appear yellow under the same circumstances. The background will appear dark pink to red. In reality, birefringent materials will be oriented many directions on the slide, so you need to make note of the red plate's axis.
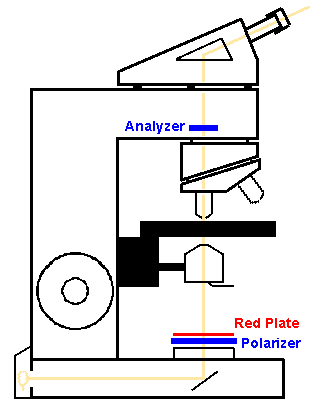
A red plate can be purchased (expensive) or you can make your own by applying two layers of clear adhesive tape to a glass slide. You will have to experiment with different tapes to get the right thickness, which you will know when your homemade "red plate" produces the reddest background. The long axis is the same as the long axis of the glass slide. Place the red plate on top of the polarizer over the light source and rotate it for the desired effect.
There are a number of usages for polarized light microscopy. These include identification of the following:
Exogenous crystalline material (most common example is talc crystals found in subcutaneous injection site, in lungs, and in organs of the mononuclear phagocyte system of persons engaging in injection drug use)
Endogenous crystalline material: crystals of sodium urate in gouty tophi, calcium pyrophosphate crystals in persons with calcium pyrophosphate deposition disease
Collagen: collagen fibrils are naturally anisotropic and polarize a dull yellow-white
Formalin-heme pigment: an artefact of poor fixation, this pigment appears as stippled black material under light microscopy, but has bright white birefringence in a stippled pattern with polarized light
Amyloid stained with Congo red dye: the structure of amyloid gives it anisotropic properties, and when stained with Congo red, it produces a characteristic "apple green" birefringence
For optimal polarized light microscopy, the microscope's light source should be made as bright as possible (remove any other filters, but be careful viewing through the eyepiece). The analyzer filter is placed over the specimen. The polarizer filter is then rotated until the light is extinguished as much as possible. If material on the glass slide has anisotropic properties, it will "bend" the light from the polarizer to pass through the analyzer and appear bright when viewed through the eyepiece. Such materials will be "birefringent".
Examples of the use of polarized light microscopy include:
- Intravenous drug use.
- Silicosis.
- Lymph node, silicoanthracosis.
- Amyloid, cardiac.
- Sodium urate crystals, negatively birefringent.
- Calcium pyrophosphate crystals, positively birefringent.
- Urine, oval fat bodies.
Fluorescence Microscopy
Some objects or materials are best viewed with fluorescent light This light is in the ultraviolet range, just below, with shorter wavelengths, light in the visible spectrum. Fluorescence makes use of a property of materials that causes them to absorb light at a shorter (ultraviolet) wavelength, exciting material to emit light at a higher (visible) wavelength. The material to be viewed must first be stained with a dye that fluoresces, unless the material fluoresces on its own. This technique provides excellent contrast, and can also highlight patterns of staining that are helpful in diagnosis.
Fluorescent light sources include halogen, mercury, and xenon gas bulbs. The halogen bulb is the cheapest, but produces the least usable fluorescent light. The mercury source is more expensive but gives good quality light for a variety of specimen types. The xenon source is the most expensive, and is rarely used in the health care setting.
The microscope must be fitted with a source for fluorescent light. Modern fluorescent microscopes use "epifluorescence" in which the fluorescent light is aimed down on top of the specimen, which is then "excited" to give off visible light which then travels back up the same optical path and then to the eyepiece. Epifluorescence is possible because of a "dichroic" mirror. This mirror has the property of passing light at one wavelength while reflecting other wavelengths. It is thus possible to place the dichroic mirror into the path of the fluorescent light source so that the fluorescent light will be reflected down through the lens to the specimen on the glass slide on the microscope stage. The fluorescent light excites the dye or material on the slide, which then gives off light at a lower wavelength in the visible range. It is this reflected light that passes back up through the lens, passes through the dichroic mirror, and is viewed through the eyepiece. A "barrier" filter is also combined with the dichroic mirror to make sure unwanted wavelengths are not passed through it.
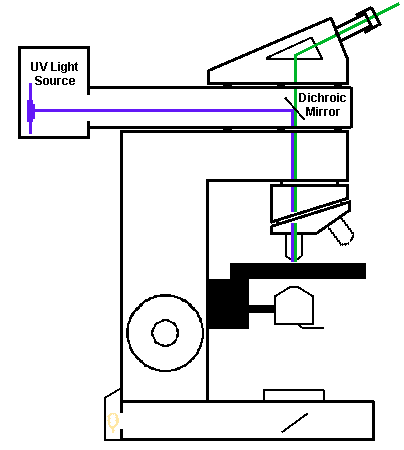
Dichroic mirrors with barrier filters can be chosen which provide optimal passage of different wavelengths. This is important, because not all fluorescent dyes will excite at the same wavelength in the ultraviolet range of light (just below the visible spectrum), which actually covers a spectrum from "ultraviolet" to "violet" to "blue-violet" to "blue" to "green".
The most common dichroic mirror will reflect the "blue" range of ultraviolet light, which excites the dye on the specimen to give off visible light that appears green. The dye fluorescein isothiocyanate (FITC) works well in this range. So does the dye auramine. The dyes methyl green pyronin and rhodamine work best in the "green" range, while thioflavins and catecholamines tend to fluoresce with light in the blue violet to violet range.
The table below indicates usages for various epifluorescence filters:
| Fluorescence Microscopy - Epifluorescence Filters
| | Range | Wavelength (Excitation) in nm | Application
|
|---|
| Green | 510 - 560 | Rhodamine, TRITC, Ethidium Bromide
| | Blue | 420 - 490 | FITC, Acridine Orange, Auramine
| | Blue-violet | 400 - 440 | Quinacrine, Thioflavine S
| | Violet | 380 - 420 | Catecholamine, Serotonin, Tetracycline
|
Examples of the use of fluorescence microscopy include:
- Mycobacteria stained with auramine.
- Antinuclear antibody test with FITC.
- Antinuclear antibody test with FITC.
- Immunofluorescence, anti-IgG with FITC, skin.
- Immunofluorescence, anti-IgG with FITC, kidney.
- Immunofluorescence, anti-IgG with FITC, kidney.
- Immunofluorescence, thioflavin, cerebrum.
Transmission Electron Microscopy
Light microscopy is limited in resolution of cellular fine structure by the wavelength of the light. Structures as small as 2 microns are about the limit of resolution. However, using a beam of focused electrons provides even more resolution, so that even higher magnifications are possible, and cellular organelles can be visualized.
The standard electron microscope consists of a long tube which contains the electron gun and the focusing apparatus in a vacuum. A magnetic coil focuses the electron beam on the specimen, acting like a microscope condenser. A "collector" magnetic coil below the specimen collects the electrons not absorbed via passage through the specimen (like a microscope lens) and passes this pattern along to a "projection" coil that enlarges the image (like a microscope eyepiece). The image can be focused on a photographic plate to obtain a print of the image, or it can be focused on a sreen for viewing. Since the prepared specimens do not preserve well, a photograph (black and white) is typically made.
Standard transmission electron microscopes have a resolving power to about 15 to 30 angstroms. Magnified images are typically from 1000X to 50,000X. By using fine grain photographic film, it is possible for further enlargements from 150,000X to 1,000,000X.
Specimens to be viewed via transmission electron microscopy are best fixed in glutaradehyde and embedded in a hard plastic material which allows sections to be cut very thin--typically a fourth of a micron for "thin" sections. The specimens can be stained with osmium tetroxide to provide additional contrast.
Examples of the use of transmission electron microscopy include:
- "Happy mitochondria".
- Premelanosomes in cells of a melanoma.
- Neurosecretory granules.
- Membranous glomerulonephritis.
Scanning Electron Microscopy (SEM)
SEM differs from standard transmission electron microscopy in that the electrons are bounced off the surface of an object with SEM, not passed through it. The major advantages of SEM are the great depth of field (more of the specimen can be in focus at once) and simple specimen preparation. The great depth of field allows incredibly detailed views of the surface of an object. Objects that are conductive (metallic) can be used "as is" but other objects to be viewed must first be prepared by coating with a very thin layer of gold so that the electrons will reflect off the surface. All samples must first be processed to remove water and volatile compounds which might vaporize in the SEM's vacuum chamber.
Like transmission EM, magnetic coils are used to focus and collect the electrons targeted on the specimen. However, with SEM, like a television, the pattern of electron deflection is collected and directed to a cathode ray tube which displays the pattern on a screen for viewing that corresponds to the surface of the sample.
In addition, a unit that detects the x-rays emitted when the electron beam strikes the sample can be added to the SEM unit. This allows x-rays to be bounced off a targeted area of the object under view. The diffraction pattern of the x-rays will provide information about the elemental composition of the targeted area.
An example of the use of scanning electron microscopy:
- Gunshot residue under SEM.
- Gunshot residue, x-ray diffraction pattern.
|
 Return to the Anatomy-Histology main menu.
Return to the Anatomy-Histology main menu.
 Return to the Anatomy-Histology main menu.
Return to the Anatomy-Histology main menu. Return to the Anatomy-Histology main menu.
Return to the Anatomy-Histology main menu.