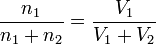Ideal gas law/Tutorials
- All gases mentioned below are assumed to be ideal, i.e. their p, V, T dependence is given by the ideal gas law.
- Absolute temperature is given by K = °C + 273.15.
- All pressures are absolute.
- The molar gas constant R = 0.082057 atm·L/(K·mol).
[edit] Example problems
[edit] Problem 1
Determine the volume of 1 mol of ideal gas at pressure 1 atm and temperature 20 °C.
[edit] Problem 2
Compute from Charles' and Gay-Lussac's law (V/T is constant) the volume of an ideal gas at 1 atm and 0 °C (Use the final result of the previous problem). Write VT for the volume at T °C, then
[edit] Problem 3
A certain amount of gas that has an initial pressure of 1 atm and an initial volume of 2 L, is compressed to a final pressure of 5 atm at constant temperature. What is the final volume of the gas?
[edit] Boyle's law (pV is constant)
or
Inserting the given numbers
[edit] Ideal gas law
The number n of moles is constant
It is given that the initial and final temperature are equal, 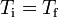 , therefore the products RT on both sides of the equation cancel, and Eq. (1.4) reduces to Eq. (1.1).
, therefore the products RT on both sides of the equation cancel, and Eq. (1.4) reduces to Eq. (1.1).
[edit] Problem 4
How many moles of nitrogen are present in a 50 L tank at 25 °C when the pressure is 10 atm? Numbers include only 3 significant figures.
[edit] Problem 5
Given is that dry air consists of 78.1% N2, 20.1% O2, and 0.8% Ar (volume percentages). The atomic weights of N, O, and Ar are 14.0, 16.0 and 39.9, respectively. Compute the mass of 1 m3 of dry air at 1 atm and 20 °C.
[edit] Answer
Since for ideal gases the volume V is proportional to the number of moles n, a volume percentage is equal to a molar percentage. For instance, for a mixture of two gases, it is easily shown that
which states that the molar percentage of gas 1 is equal to the volume percentage of gas 1.
The mass of 1 mole of dry air is
- M = 0.781×28.0 + 0.201×32.0 + 0.008×39.9 g = 28.6192 g
In problem 1 it is found that the volume of 1 mole of ideal gas at 1 atm and 20 °C is 24.0550 L = 24.0550×10−3 m3, or
- 1 m3 contains 1/(24.0550×10−3) = 41.5714 mol
Hence the mass of 1 cubic meter of dry air is
- M = 28.6192 × 41.5714 = 1189.7 g = 1.1897 kg


![V = \frac{n\,R\,T}{p} = \frac{1\cdot 0.082057\cdot (20+273.15)}{1} \quad\left[ \frac{ \mathrm{mol}\cdot\frac {\mathrm{atm}\cdot\mathrm{L}} {\mathrm{K}\cdot\mathrm{mol}} \cdot\mathrm{K} } {\mathrm{atm}} \right] = 24.0550 \quad [\mathrm{L}]](../../w/images/math/5/7/b/57bb8cf285eac4fa1e13162ce7f986ce.png)
![\frac{V_{20}}{273.15+20} = \frac{V_0}{273.15+0} \quad\Longrightarrow V_0 = 273.15 \times \frac{24.0550}{293.15} = 22.4139\; \;[\mathrm{L}]](../../w/images/math/2/9/8/2980450d797bbfc81876b8aca8b579f9.png)

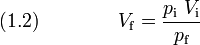
![(1.3)\qquad\qquad V_\mathrm{f} = \left(\frac{1\cdot 2}{5}\right)\;\left[ \frac{\mathrm{atm}\sdot\mathrm{L}}{\mathrm{atm}} \right] = 0.4\; [\mathrm{L}]](../../w/images/math/4/9/7/49772c31dca9a82c51a84c8877eb553f.png)
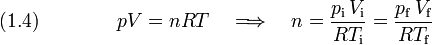
![n=\frac{p\,V}{R\,T} = \frac{10.0\cdot 50.0} {0.0821 \cdot (273+25.0)} \quad \left[ \frac{\mathrm{atm}\cdot \mathrm{L}}{\frac{\mathrm{atm} \cdot \mathrm{L}}{\mathrm{K}\cdot \mathrm{mol}}\cdot\mathrm{K}} \right] =\frac{500}{0.0821 \cdot 298}\quad\left[ \frac{\mathrm{mol} \cdot \mathrm{atm}\cdot \mathrm{L}}{\mathrm{atm}\cdot \mathrm{L}} \right] = 20.4 \quad [\mathrm{mol}]](../../w/images/math/9/9/4/994a9f620b8dcde176795fe0a2dc324d.png)