Examination of Gunshot Residue
Composition of gunshot residue
Firing a weapon produces combustion of the primer and powder of
the cartridge. The residue of the combustion products, or unburned primer or
powder components, can be used to detect a fired cartridge. Residue may be
found on the skin or clothing of the person who fired the gun, on an entrance
wound of a victim, or on other target materials at the scene. The discharge of
a firearm, particularly a revolver, can deposit residues even to persons at
close proximity, so interpretations as to who fired the weapon should be made
with caution. (Thornton, 1986) The major primer elements are lead (Pb), barium (Ba), or antimony (Sb). Usually, all three are present. Less common elements include aluminum (Al), sulfur (S), tin (Sn), calcium (Ca), potassium (K), chlorine (Cl), or silicon
(Si). A mercury-fulminant based primer may be found in ammunition manufactured in Eastern Europe and used in the Middle East.(Zeichner, et al, 1992) Primer elements may be easier to detect in residues because they do not get as hot as the powder, and compounds (not just elements) may be detectable. (Tassa et al, 1982b) In addition, primer residues may adhere to fired bullets and gradually
ablate through the path of the bullet. Thus, primer residue may be found in
targets or wounds at considerable distance from the muzzle (up to 200 meters). The cartridge case, bullet, bullet coating, and metal jacket also
contain specific elements that can be detected. Virtually all cartridge cases
are made of brass (70% copper and 30% zinc). A few have a nickel coating.
Primer cases are of similar composition (Cu-Zn). Bullet cores are most often
lead and antimony, with a very few having a ferrous alloy core. Bullet jackets
are usually brass (90% copper with 10% zinc), but some are a ferrous alloy and
some are aluminum. Some bullet coatings may also contain nickel. (Ravreby,
1982). Modern gunpowder, or "smokeless" powder, can contain up to 23
organic compounds (FBI study). Nitrocellulose is virtually always present,
along with other compounds containing nitrate or nitrogen. One of these
compounds, diphenylamine (used as a stabilizer in the powder), can be detected
using reagents containing sulfuric acid. (Maloney et al, 1982) Modern
gunpowders are also described as "single-base" when the basic
ingredient is nitrocellulose and as "double-base" when there is
additionally 1 to 40% nitroglycerine added. Hardy and Chera (1979) describe a
method to differentiate them using a mass spectrometer. In the physical examination of the scene or body for evidence of
gunshot residue, it must be remembered that lead residues may mimic gunshot
residue. Lead residues may be found up to 30 feet from the muzzle, and are
always present on the opposite side of a penetrated target. Such a situation
has been reported when an intermediate target (glass) was present. (Messler and
Armstrong, 1978) Though the amount of residue deposited tends to decrease with increasing range of fire, the actual deposits can be highly variable for ranges up to 20 cm.(Brown, Cauchi, et al, 1999)
Detection of Gunshot Residue
The major methods for detection of primer residues are neutron
activation analysis (NAA), atomic absorption spectrophotometry (AAS), and
scanning electron microscopy with energy dispersive analysis (SEM-EDA). For
these methods, samples must be obtained from the skin surfaces of a victim at
the scene. Delay in obtaining residues, movement, or washing of the body prior
to autopsy will diminish or destroy gunshot residues. (Kilty, 1975) Scanning electron microscopy with energy dispersive analysis (SEM-EDA)
has become an excellent method for detection of gunshot residue. (Andrasko and
Maehly, 1977) The method of collection for residue is quite simple and easily carried out in the field (Tassa, et al, 1982a) directly onto the gummed surface of a chuck, or holder, applied to the surface (skin or other material) to be tested. The chuck, with the residue on the surface, can be directly prepared for examination in the SEM device. A polyvinyl-alcohol (PVAL) collection method has been developed that has the advantage of preserving the topical distribution of gunshot residues as well as sampling of other trace materials such as blood.(Schyma and Placidi, 2000)
A major advantage of this method is that SEM can reveal the actual
surface details of the particles examined, for comparison with known examples of
gunshot residue, and pictures can be taken. The large particles of partially
burned powder and the spheres of residue can be distinguished from contaminant
materials.
Scanning Electron Micrograph of GSR
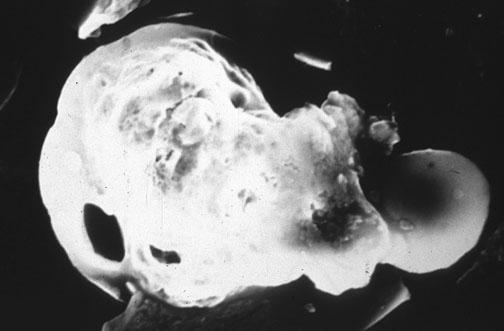
An X-ray analyzer can be beamed directly onto the particles, so that
the energy dispersive pattern (EDX) can be generated, giving the elemental composition of the particles. (Nesbitt et al, 1976) A computer program to speed up the search for GSR particles by SEM has been described (Tillman, 1987)
Diagram of the SEM-EDX pattern of GSR
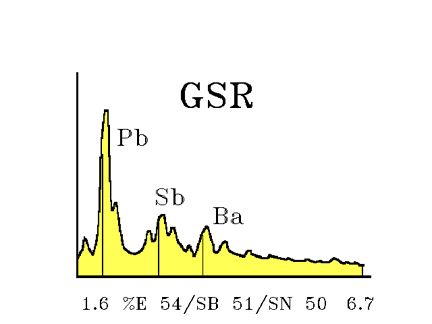
It should be remembered that any hand or body part that was close to
the fired weapon may have residue appearing consistent with having fired the
weapon (Thornton, 1986). Clothing should always be retained on the body up to
autopsy, as this may modify entrance wounds, need examination for gunshot
residues, or aid in interpretation of the scene. Gunshot residue analysis requires careful evaluation. False positives
may be caused by contamination or transfer of GSR to the body by mishandling, or
when the body is heavily contaminated by GSR from previous shooting. False
negatives result from washing of the hands (when this area is sampled) or by
victim wearing gloves. A rifle or shotgun may not deposit GSR on hands. SEM may also have usefulness for examination of bullets, as embedded
materials from the target such as bone fragments may aid in reconstruction of
the scene (DiMaio VJ et al, 1987). SEM has been used to study tool marks made
by the firing pin impressions in the primers of spent cartridges. Such findings
could be useful to determine which gun was used to fire the cartridge. Grove et
al (1972) found that SEM could reveal clearly all surface detail in the
impression and that 50% of shotgun impressions and 75% of rifle impressions
could be positively identified on the basis of four or more individual
characteristics, given similar class characteristics. It may be difficult to both find and determine the nature of gunshot
wounds in a decomposed body. Determination of the range may be particularly
difficult. Extreme care should be taken to avoid misinterpretation of the
wounds and artefacts.
Other Examinations
Sometimes the question of whether the victim was holding the
firearm arises in investigation. Lee (1986) has described an improved method
for the detection of iron traces on the hands by use of a ferrozine spray.
Prior to this, a hydroxyguinoline test was employed, but required fluorescent
photography. (Stevens and Messler, 1974) Cases have been described in which suicide victims' hands were stained
orange-brown from contact with gun barrels following death, presumably from
perspiration coupled with a prolonged post-mortem interval of contact. (Norton
et al, 1979) Latent fingerprints may be detectable on cartridges and expended shell
casings. Such fingerprints, called latent because they are transferred via a
substance on the skin ridges to an object. On a gun, such substances could
include cleaning solvents or gun oils. Usually, the substances consist of
perspiration mixed with oils from sebaceous glands. Conditions of increased
temperature and low humidity decrease the persistence of fingerprints. Brass
retains the fingerprints better than nickel-plated materials. (Given, 1976) Each firearm sold (other than black powder weapons) has a
manufacturer's serial number stamped into it which may be used to identify the
weapon. Registration of firearms provides a way of tracing gun ownership.
However, attempts may be made to obliterate registration numbers by grinding or
filing the metal. (Polk and Giessen, 1975) Gas chromatography has been used to identify gun oils in targets, and
was very sensitive, even with stored specimens (Kijewski and Jakel, 1986).
|
 Return to the Firearms Tutorial menu.
Return to the Firearms Tutorial menu. Return to the Firearms Tutorial menu.
Return to the Firearms Tutorial menu. Return to Criminalistics Laboratory Methods.
Return to Criminalistics Laboratory Methods.

 Proceed to Other Issues and Injuries.
Proceed to Other Issues and Injuries.