|
A 68-year-old woman has noted increasing difficulty with breathing for the past 4 years. She has become increasingly fatigued. She has had episodes during the past 6 months of waking up at night with marked shortness of breath, coughing up frothy sputum.
A chest radiograph shows cardiomegaly, blunting of the costophrenic recesses, prominent pulmonary arteries, and peripheral lower lobe opacifications.
On physical examination vital signs include T 37.0 C, P 82/min, RR 20/min and labored (but without use of accessory muscles), and BP 155/90 mm Hg. She has poor peripheral capillary filling and cold hands. There is pitting edema to the knees. There is jugular venous distension while sitting to the angle of the jaw. Auscultation of the chest shows a holosystolic murmur and diminished S1 and S2. Diffuse bilateral coarse inspiratory crackles are heard over both lung bases. Her liver edge is palpable, but the spleen is not palpable.
Laboratory studies show Na 139 mmol/L, K 3.9 mmol/L, Cl 101 mmol/L, HCO3 28 mmol/L, glucose 124 mg/dL, creatinine 1.4 mg/dL, and urea nitrogen 25 mg/dL.
An EKG is performed. Review the features of a normal EKG.
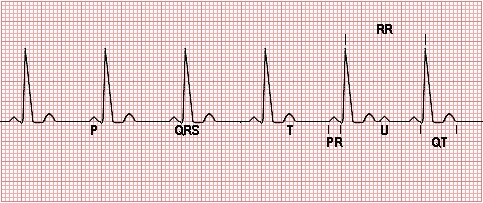
Each small square represents 0.04 second horizontally and 0.01 millivolt vertically.
The P wave represents depolarization of the atria.
The QRS complex represents bilateral ventricular depolarization. The QRS duration is normally 0.06 - 0.10 seconds and represents the duration of ventricular muscle depolarization.
The PR interval is the time interval from the onset of atrial depolarization (P wave) to the onset of ventricular depolarization (QRS complex). The normal PR interval is 0.12 - 0.20 seconds.
The ST-T wave represents ventricular repolarization.
The QT interval (QTc) represents the duration of ventricular depolarization and repolarization and is normally < 0.40 seconds at a heart rate of 70/min or above.
The U wave, if present, represents "after depolarizations" in the ventricles.
The RR interval is the duration of the ventricular cardiac cycle, an indicator of the ventricular rate.
An EKG is performed in this patient which shows a regular rate and rhythm but increased amplitude in lead II.
Cardiac catheterization:
- Chamber volume at end of diastole = 0.138 L
- Chamber volume at end of systole = 0.083 L
- Heart rate = 91/min
- Left ventricular end-diastolic pressure 12 mmHg
- Pulmonary arterial pressure 50/20 mm Hg, mean 30 mmHg
- Pulmonary artery wedge pressure = 20 mm Hg
- Right atrial pressure = 12 mm Hg
- Coronary arteries show <20% stenosis
Questions:
1.1 What is her stroke volume?
1.2 What is her cardiac output?
1.3 What is her cardiac index (her body surface area is 1.6 m2)?
1.4 What is her ejection fraction?
1.1 Stroke Volume = ((Chamber Volume at End Diastole) - (Chamber Volume at End Systole))
0.055 L
1.2 Cardiac Output = (Stroke Volume) X (Heart Rate)
5 L/min
1.3 Cardiac Index = (Cardiac Output) / (Body Surface Area)
3.12 L/min/m2
1.4 Ejection Fraction = ((Stroke Volume) / (Chamber Volume at End Diastole)) * 100
39.85%
The ejection fraction (EF) is the amount of blood ejected from the left ventricle (LV) with each heart beat and is normally > 55%. An EF can be calculated with an echocardiogram, a left ventriculogram done during a cardiac catheterization, or a nuclear study called a MUGA. The method of calculating the EF involves tracing the dimensions of the LV at the end of its contraction period (systole) and at the end of its relaxation period (diastole). These dimensions are then used to calculate the EF with a standard mathematical formula. Many cardiologists visually estimate the EF. Generally, the calculated EF is more accurate. The lower the EF, the worse the congestive heart failure.
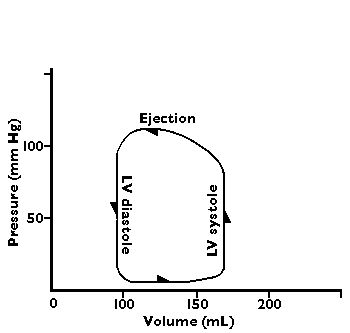
A flow-volume loop diagram of normal cardiac output has the following appearance:
In the diagrams below, abnormal flow-volume loops are shown:
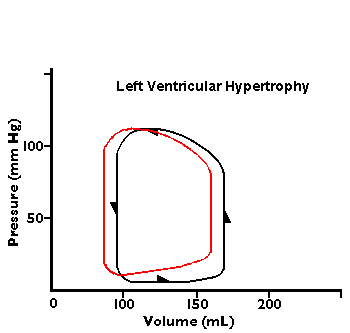
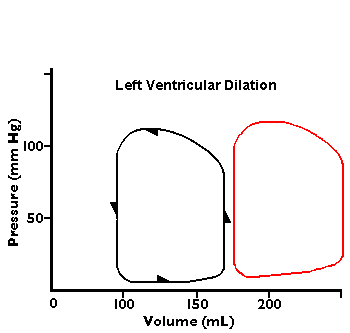
1.5 Compare these cardiac catheterization findings to normal.
She has a decreased cardiac output with decreased ejection fraction with congestive heart failure.
1.6 What determines the voltage that is recorded on the limb leads? Mention several factors.
Voltage is determined by the electrical axis and current. The amplitude of a deflection (such as a QRS with ventricular depolarization) will be greatest in the lead in which the axis of depolarization is progressing near the lead being measured, such as lead II. The voltage will be increased with increasing muscle mass. The voltage will be decreased if the leads are not attached properly.
1.7 What sorts of things lengthen the PR interval? Can you suggest how they might work?
A short PR of < 0.12 seconds may be due to preexcitation syndromes such as the Wolff-Parkinson-White (WPW) Syndrome in which an accessory pathway (called the "Kent" bundle) connects the right atrium to the right ventricle ,or the left atrium to the left ventricle, and permits early activation of the ventricles, producing a short PR interval.
A prolonged PR of >0.20 seconds may be caused by first or second degree AV block.
1.8 Does she have right or left heart disease or both?
She has evidence for both right and left heart failure. The pulmonary "crackles" (once termed "rales") result from the presence of fluid in alveoli, with bubbles popping, particularly with inspiration, and this is indicative of left heart failure. The lungs filling with fluid, particularly when recumbent, explains her orthopnea.
Pure right heart failure (cor pulmonale) results from obstructive or restrictive lung diseases (emphysema, sarcoidosis, silicosis, etc) that reduce the pulmonary vascular bed. Pulmonic stenosis can do it as well.
Pure left heart failure is most often due to systemic hypertension, but aortic valvular disease can also do it.
Cardiomyopathies can lead to global four chamber dysfunction.
A big heart is a bad heart.
1.9 What cardiac problem is causing these findings?
She has reduced cardiac chamber compliance. The lack of compliance of heart muscle reduces cardiac output.
Patients with diastolic dysfunction, compared to those with systolic dysfunction, have normal or often enhanced contractile function of the left ventricle, and there is a normal increase in stroke volume with an increase in preload. Since the ventricle in these patients can increase cardiac output, there should be no mismatch between demand for more cardiac output and the ability of the left ventricle to supply it. Nevertheless, patients with diastolic dysfunction have dyspnea and fatigue, particularly with exercise or stress, just like patients with impaired systolic contractile function because there is reduced ventricular compliance with impairment of ventricular function so that the heart is unable to use preload reserve, since high cardiac filling pressures occur at normal or decreased intraventricular volumes.
The figure below shows the relationship between ventricular pressure and ventricular volume. Curve (A) demonstrates the change in pressure that occurs with a given change in volume throughout diastole. Curve (B) shows an increase in chamber compliance. Curve (C) shows a decrease in chamber compliance, and at any given ventricular volume, there is an increased ventricular pressure. Decreased compliance also results in a more rapid increase in ventricular pressure for a given change in volume and a steeper slope of the pressure-volume curve. Increased stiffness (decreased compliance) shifts the pressure-volume curve to the left, producing a higher pressure for a given diastolic volume. Changes in compliance can be due to several factors influencing diastolic function such as myocardial or endocardial fibrosis, ischemia, valvular, and hypertensive heart disease.
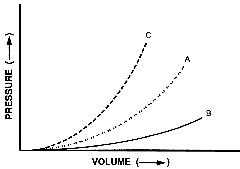
The ventricular diastolic pressure - volume relationship may be abnormal because of changes in active relaxation, passive compliance factors or both. Left ventricular diastolic failure leads to impaired ventricular filling with high left atrial and pulmonary venous pressures. The most likely reason for this is elevation of pulmonary venous pressures as a result of elevated ventricular pressure, reflected on the left atrium due to decreased ventricular compliance. Stroke volume is limited due to reduced ventricular preload.
If systolic left ventricular failure, from valvular disease or hypertension, is superimposed upon diastolic dysfunction, then the effect is exaggerated. A change in the patient's symptoms or exercise tolerance may suggest such decompensation.
What effect might loss of right atrial compliance have on production of atrial natriuretic peptide-factor-hormone?
ANF is primarily released with atrial stretching. With loss of compliance, there is less stretching.
1.10 What therapies are available?
Diastolic dysfunction may remain asymptomatic for several years, but early detection and treatment will help prevent ventricular myocardial damage and decrease the risk for systolic dysfunction. However, no single drug has exclusive lusitropic properties, i.e. increase relaxation of the heart in diastole, which enhance myocardial relaxation without negative effects on ventricular contractility. Therefore, there are four treatment therapies advocated for the treatment of diastolic dysfunction:
A. Reduction of central blood volume with diuretics
Diuretics help reduce pulmonary congestion by shifting the pressure-volume curve downwards. This positive effect on chamber stiffness reduces systemic blood volume and lowers right atrial blood volume. Diuretics require careful monitoring because of the sensitivity of some patients to cardiac volume. Excessive diuresis may lead to a sudden drop in stoke volume and cardiac output.
Angiotensin receptor blockers (ARBs) may be used in patients who are intolerant of ACE inhibitors due to cough or angioedema.
B. Improvement of left ventricular relaxation with calcium channel blockers and ACE inhibitors
Calcium channel blockers, though not generally used-just in special cases, may improve myocardial relaxation and enhance diastolic filling. These drugs seem to be best matched to the pathophysiology of relaxation disturbances due to their ability to decrease calcium concentration within cells and also reduce afterload. Calcium channel blockers with negative dromotropic (heart rate) action such as verapamil or diltiazem may improve diastolic filling by a reduction in heart rate. They have also been shown to reduce muscle mass in patients with hypertension, thus, improving the elastic properties of the myocardium. Calcium blockers are first line drugs in patients with hypertrophic cardiomyopathy (a rare disease) due to their beneficial effect on relaxation and diastolic filling which are often severe in these patients.
C. Regression of left ventricular hypertrophy and decrease in wall thickness and removal of excess collagen by ACE inhibitors or spironolactone.
D. Maintenance of atrial contraction and control of heart rate -- beta-blockers and antiarrythmics
Beta-blockers have been used for many years to control blood pressure and, thus, myocardial hypertrophy. Slowing of the heart seems to be the primary benefit and not improvement of isovolumetric relation as once thought. Beta-blockers also cause regression of left ventricular hypertrophy, which will also improve diastolic filling.
1.11 If the patient had a blood pressure of 165/100 mm Hg, what pharmacologic therapy would you consider? Use your PDA to find specific agents and dosing regimens that the patient could reasonably follow.
Initial theapy is usually either a thiazide diuretic or an ACE inhibitor. If one alone is not sufficient to lower blood pressure adequately, then both are usually prescribed. If control of blood pressure is still not adequate with this combination, then another pharmacologic agent may be added, and this third agent is usually a beta-adrenergic blocker. If adding this third drug is still not sufficient, then an alpha-1 blocker or alpha-2 agonist may be used.
| Drug | Dose
| | Hydrochlorothiazide | 12.5-50 mg PO qd
| | Furosemide | 20-320 mg PO bid
| | Captopril | 25-50 mg PO tid
| | Lisinopril | 10-40 mg PO qd
| | Enalapril | 5 mg PO qd
| | Propranolol | 80-240 mg PO bid
| | Timolol | 20-40 mg PO bid
| | Atenolol | 50-100 mg PO qd
| | Labetalol | 200-800 mg PO bid
| | Losartan | 25-50 mg PO qd
| | Clonidine | 1-3 mg PO bid
| | Prazosin | 2-20 mg PO qd
| | Amlodipine | 2.5-10 mg PO qd
| | Hydralazine | 50-200 mg PO bid
|
| 


