Case Presentation:
- History: A 61 year old man is cleaning out a fence corner in the pasture that contains old rusting cans, drums, and tools, along with rolls of rusting barbed wire. He incurs a deep puncture wound to his right arm. Over the next week, the wound heals over, but remains tender, slightly red, and mildly swollen. Ten days following the injury he develops diaphoresis, difficulty swallowing, and back spasms. He has difficulty speaking because of sustained contraction of facial muscles. He then seeks medical attention. The wound on his arm is debrided, and a wound culture is obtained.
- What condition does he have?
- Tetanus.
- What does the wound culture sample show on gram stain and culture?
- The gram stain shows gram positive rods with terminal spores, giving the organism a "drumstick" appearance. The culture grows Clostridium tetani.
- What is the pathophysiology of his illness?
- C. tetani produces a neurotoxin called tetanospasmin which binds irreversibly to nerve endings and blocks release of inhibitory synapse neurotransmitters such as GABA. This results in overexcitation with muscle spasm. It takes a few days to a few weeks for symptoms to appear. C. tetani is found in the environment.
- How could this disease have been prevented?
- Initial treatment of the wound and antibiotic therapy would have helped. However, "active" immunization will prevent the neurologic complications. Tetanus immunization is commonplace in developed nations, and few cases are reported. For persons who have developed tetanus, "passive" immunization with administration of human tetanus immunoblobulin will help to neutralize remaining toxin.
- How do antibodies provide a protective function?
- The body's only immediate defences against attack by micro-organisms include the innate immune system and pre-formed antibodies. Circulating antibodies are able to neutralize microbial agents before they begin to proliferate and spread. The humoral immune response to make more specific antibody takes a week. Approximately 3 gm of immunoglobulin is made each day in tissues of the body and circulates, is excreted, or eventually decomposes.
- There are several ways to develop these preformed circulating antibodies:
- Congenital: Everyone who is born has antibodies acquired transplacentally prior to birth. Antibodies are also acquired from breast milk. These antibodies provide a degree of immunity in infancy.
- Encounter with infectious agents: A primary immune response leaves some plasma cells behind that secrete low levels of antibody for months to years.
- Immunization (vaccination): All or part of an organism or its toxin is introduced under controlled circumstances to the body's immune system so that infection does not occur, but a primary immune response does.
- How do antibodies function in infection?
- Antibodies can coat micro-organisms and/or their toxins, and this makes them more susceptible to removal by phagocytic cells. Once coated, the Fc portions of antibodies can activate macrophages, neutrophils, eosinophils, NK cells, and complement.
- Neutralizing antibodies bind to infectious agents and render them incapable of infecting host tissues. Neutralizing antibodies render toxins ineffective.
- Opsonizing antibodies bind to infectious agents and render them susceptible to phagocytosis. This primarily occurs because the Fc portions of the antibodies are projecting outward. Opsonized organisms are easily removed by phagocytosis, particularly in the spleen, which has large numbers of fixed phagocytes lining sinusoids that grab antibody-coated particles flowing by. Neutrophils can also ingest antibody-coated materials.
- Infectious agents can resist humoral mechanisms by varying their surface macromolecules, such as glycoprotein coats. Resistant strains can become selected by the antibody response.
- Describe the structure and function of an antibody.
- Antibodies are immunoglobulins. Immunoglobulins consist of four protein chains: two identical heavy chains and two identical light chains. The chains are held together with disulfide bonds and form a rough "Y" shape. The bottom of the "Y" consists of the two bound heavy chains. A light chain attaches to each heavy chain on the arms of the "Y". The bottom of the "Y" is called the Fc region. The arms of the "Y" form the Fab region. Each heavy and light chain has a constant and a variable region. The variable regions are at the end of the "Y" arms in the Fab portion. It is the variable regions that give the antibody its specificity for a particular antigen.
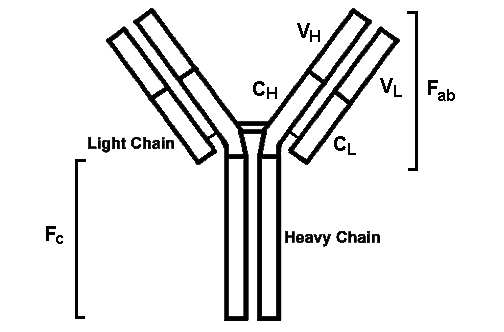
- Each variable region of a chain has three "hypervariable" regions called CDRs. These regions provide great variability in antibody specificity and also provide for tight binding to an antigen. Thus, the specificity of an antibody is provided by the Fab portions, while the Fc portion gives the antibody most of its biologic properties. The Fc portion also binds to cells that have Fc receptors.
- Infectious agents typically have lots of antigenic components, and each antigen is also complex. An antibody typically recognizes just a part of an antigen. These parts are known as epitopes, or determinants. An antibody made to one particular epitope may also exhibit binding, though somewhat weaker binding, to another epitope that is structurally similar. This is known as cross reactivity.
- The strength of binding of the Fab portion to the epitope is known as affinity. As antibodies are made in immune responses to an infectious agent or its toxins, the quality of the antibody tends to improve, with increasing affinity. This process is known as affinity maturation. The total strength of antigen-antibody binding, not just one interaction, is called avidity.
- When antibodies are made to an infectious agent, typically multiple antibodies to multiple epitopes are produced, so there is variabilty, and different clones of B cells are involved in antibody production. Thus, the antibody produced is polyclonal. If only a single clone of B cells produces antibody, then the antibody is monoclonal. Monoclonal antibody is abnormal, and typically represents a neoplastic response. Monoclonal antibodies are manufactured commercially to be used in diagnostic testing and in some treatment methods.
- What types of antibodies are produced?
- Different Ig heavy chain classes impart different functions to antibodies:
- IgG is best for neutralization of infectious agents, opsonization of antigens, activation of the classic complement pathway, and activation of NK cells.
- IgM is the first immunoglobulin secreted in a primary response. It can activate the classic pathway.
- IgA is mainly secreted into the gastrointestinal and respiratory tracts to provide "mucosal immunity".
- IgE is typically bound to mast cells and mediates cytotoxicity by eosinophils in allergic responses and in parasitic infections.
- Different Ig light chains are produced that function similarly:
- Kappa
- Lambda
- How is it possible that B cells can make antibodies that react with so many potentially different infectious agents?
- During development of the immune system, as the B cells were proliferating, they had genes that determined the makeup of immunoglobulin production. These genes consisted of variable (V), diversity (D), and joining (J) genes that underwent recombination to produce extensive variability in the variable regions of the immunoglobulins. The multitude of VDJ combinations in the B cells provides for tremendous diversity and the ability of B cells to respond by producing antibody to any possible antigenic challenge. The number of possible combinations is 1011.
- What is the role of complement?
- Complement is activated via either the classic or alternative pathways. Complement components are always circulating in the bloodstream. They are continually being produced and removed.
- The most abundant component is C3. As C3 breaks down, a C3b component is generated. It is this component that can attach to infectious agents and trigger additional complement component activation. This is the alternate pathway.
Animation: alternate pathway of complement activation
- The binding of IgG (subclasses 1 or 3) or IgM to an infectious agent triggers the activation of complement component C1, which becomes activated when it can crosslink two bound Fc regions. This is the classic pathway for complement activation. The C1 binds C4 and C2, which together activate C3, joining where the alternate pathway starts.
Animation: classic pathway of complement activation
- In addition, circulating mannose binding lectin can bind to micro-organisms, and this sets off the classic pathway. This is known as the lectin pathway.
- Regardless of the initial pathway of activation, the immediate result is generation of C3b that coats infectious agents. This renders them susceptible to phagocytosis by phagocytes that have complement receptors. In essence, coating of the organisms by C3b is similar to opsonization by antibody. Thus, C3b acts as an opsonin.
- Activation of complement component C5 by the complex of C3b, C4b, and C2a results in formation of C5a, which has chemoattractant properties, and C5b, which stimulates formation of the "membrane attack complex" made up of components C6,7,8, and 9. This membrane attack complex punches holes in the cell that initiated the complement cascade.
- Normal host cells have inhibitors of complement activation. There are circulating inhibitors (C1 inhibitor) and proteolytic agents that mitigate complement effects.
- What is the purpose of vaccination?
- The purpose of immunization (vaccination) is the artificial induction of a protective immune response that will provide circulating antibody that will help to prevent a real infection from occurring. Of course, surviving the "real thing" imparts immunity, but carries a risk for morbidity/mortality. Aside from the benefit to an individual, immunization has benefits for a population as a whole. If enough persons are immunized, then the ability of an infectious agent to spread amongst persons is diminished. Thus, "herd" immunity mitigates against epidemics.
- Instead of using the "real" infectious organisms, the strategy with vaccines is the use of an agent that cannot cause disease, but can stimulate the immune system to produce specific antibodies. Strategies include the use of live but "attenuated" strains of the infectious agent, or "inactivated" vaccines. An infectious agent or its toxin can be inactivated by heat or chemical treatment to render it harmless. The three major types of inactive vaccines are:
-
Toxoids - inactivated toxins
-
Killed agents - inactivated bacterial or viral organisms
- Subunits - capsule or protein subunits of bacteria or viruses
- In general, in order to produce a cell mediated immune response, more useful in viral infections, a live attenuated vaccine must be given
- The "inactivated" types of vaccines tend to be less effective, requiring larger doses and periodic "booster shots" to maintain humoral immunity. They are generally used for protection against bacteria or bacterial toxins.
- What is the difference between "active" and "passive" immunization?
- With "active" immunization, the vaccine is given prospectively, in order to initiate an immune response, with two purposes: (1) preventing possible disease in the individual given the vaccine, and (2) creating a "herd" immunity in multiple individuals that makes spread of infection among persons less likely, preventing an epidemic.
- With "passive" immunization, an immune globulin preparation containing preformed antibodies is given to an individual at the time an infection occurs with two possible purposes: (1) preventing the infection from taking hold completely, or (2) minimizing damage from the infection. Immune globulin also may be given to persons who have poorly functioning immune systems (e.g., persons who are immunocompromised).
Case Presentation:
- History: An ultrasound is performed at 18 weeks and shows a fetus that is small for gestational age. Organs of the thorax and abdomen are normally formed. However, the brain shows areas of periventricular leukomalacia (degeneration of white matter) with areas of brightness around slightly dilated ventricles. A "TORCH" assay is done on maternal serum and shows:
-
| Toxoplasma |
|
| IgM 0.05 TIV |
(0.90 - 1.10 equivocal; >1.10 positive) |
| IgG 4 IU/mL |
(6 - 200 equivocal; >200 positive) |
| Rubella |
|
| IgM 0.47 RIV |
(0.90 - 1.10 equivocal; >1.10 positive) |
| IgG 20 IU/mL |
(10 - 15 equivocal; >15 positive) |
| Cytomegalovirus |
|
| IgM 2.30 EU |
(0.90 - 1.10 equivocal; >1.10 positive) |
| IgG 1.56 IV |
(0.90 - 1.09 equivocal; >1.09 positive) |
| Herpes simplex virus types I and II combined |
|
| IgM 0.81 IV |
(0.91 - 0.99 equivocal; >1.00 positive) |
| IgG 0.82 IV |
(0.80 - 0.99 equivocal; >1.00 positive) |
The baby is delivered stillborn at 26 weeks gestation. There is mild fetal and placental hydrops (edema). There is mild hepatosplenomegaly. The heart is slightly enlarged. No congenital anomalies are noted. The brain is soft and shows chalky white areas with calcification around the cerebral ventricles. Microscopically, the placenta shows a chronic villitis.
- What is affecting the fetus?
- Cytomegalovirus infection.
Congenital Cytomegalovirus Infection in a Fetus
- Explain the pathophysiology of the TORCH assay.
- If the mother does not have protective antibodies against an infectious agent, and that agent can cross the placenta, then the fetus can become infected. Serologic evidence of congenital infection can be done in the form of TORCH titers on maternal or neonatal serum. The presence of an elevation in the IgM titer over the IgG titer suggests recent infection. A high titer of IgG, but not IgM, suggests past maternal infection. Many persons (50 to 80% of adults) have had subclinical CMV infections and the only evidence is serologic. She probably received a rubella vaccination in the past to account for the presence of rubella IgG. Many persons have been exposed to herpes simplex viruses, and in this setting the equivocal IgG is of no major importance.
- How do protective antibodies reach the fetus?
- There is a neonatal Fc receptor in the placenta (a fetal tissue) which aids in active transport of IgG across the placenta to fetal circulation. Following birth, a source of antibodies for the infant is breast milk, which contains colostrum that has immunoglobulins. Neonatal intestinal epithelial cells have Fc receptors that facilitate transport of ingested immunoglobulins across the mucosa.
Case Presentation:
- History: A 5 year old child has developed a fever to 38.4 C over the past two days. The child is eating poorly because of discomfort and difficulty swallowing. The child seems listless. There is facial swelling and tenderness bilaterally in the region anterior and below the ears. These problems diminish over the next week, but then the child develops a stiff neck with drowsiness and complains of headache.
- What is this condition?
- Mumps virus infection with parotitis, followed by meningitis. Meningitis is not common with mumps, but can occur, and most patients get over it without sequelae. Mumps is a paramyxovirus with RNA in its core.
- Why does this disease not occur frequently in the U.S.?
- Childhood vaccicnation with MMR (mumps, measles, rubella) has provided immunity to most children. Mumps vaccine was introduced in 1967, and since then reported cases have been reduced by >99%.
- What does the vaccine, or the primary infection, do for the immune system?
- Exposure to the antigen results in a primary immune response that is mediated predominantly by CD8 cells, because of the intracellular nature of the virus.
- Evidence for prior infection or vaccination can be demonstrated by skin testing.
- What cell population would be seen in cerebrospinal fluid obtained from lumbar puncture?
- Lymphocytes
|