Tal's Advanced Analythical Chemistry Page - For Students
Index
How to analyze non-volatiles with GC
Questions and answers (Updated from time to time)
How to Study for the Final Exam - Tips (Updated from time to time)
Recommended Sites back up back to Tal's main page
I recommend you read this short Overview of Chromatography.
LDR - Linear Detection Limit back up back to Tal's main page
I've noticed some misunderstanding regarding this term, I will try to explain:
In the booklet you received you have the Characteristics of GC detectors in figure 10. In order to get the MAXIMUM linear detection limit you simply have to multiply the MDL and the LDR.
For example, FID is linear up to 50μg C/sec.
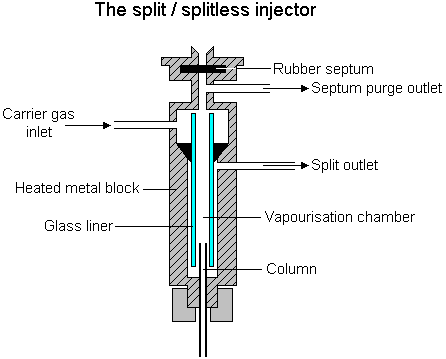
Split/Splitless back up back to Tal's main page

This kind of injector is usually fully software controlled - no hardware changes are required to define it's properties.
When our desired method is 'split', we define in our GC program that we want to work in this method and specify the 'split-ratio'.
the 'split-ratio' defines the ratio between the matter that will get into the column and the matter that is thrown out through the split-vent. A ratio of 9:1 means that the flow-rate is 9 times higher through the split-vent than through the column (if we keep the column flow-rate constant , the GC will automatically change the total flow rate to allow split changes).
When our desired injection method is 'splitless' we need to specify several constants:
1) The time when the injector will return to 'split' mode (there is usually no situation when the split valve is closed during the whole analysis).
2) The split-ratio we'll have when the injector will pass to split mode.
Usually during idle time (when the GC is not used) the split valve is open, therefore if we want to work in splitless mode we will have to tell the GC to get into splitless mode before we inject our sample (In the laboratory next semester you will press a button called 'prep-run' to do just that).
we can use the 'Split' method when our analytes have high concentration in the sample.
the 'splitless' method is usually for trace analysis (low concentrations).
A simple thing about TCD back up back to Tal's main page
Due to the TCD's many flaws, it is almost always better to use another detector if it's possible. For example, if you want to detect Ethanol and Benzene, don't think about using the TCD since FID will do a good job.
How to analyze non-volatiles with GC back up back to Tal's main page
Sometimes we want to analyze the volatiles present in a non-volatile matrix (for example analyzing a human hair for cocaine or a leaf for a pesticide). We obviously cannot directly inject that kind of sample, since (if we'll find a way to inject it) most of it will stay in the injector and will never get out - ruining the liner and our day.
Human ingenuity came up with several solutions, one of them is thermal desorption:
Thermal Desorption -
Gas-phase samples can be collected and concentrated onto adsorbent tubes from the atmosphere, or from the headspace over liquid or solid samples, and volatiles trapped onto the tubes are thermally desorbed and introduced onto the GC column. Direct thermal extraction of volatiles and semivolatiles in solid materials allows simplified trace GC analysis of volatiles and semivolatiles in such samples as polymers, waxes, powders, pharmaceutical formulations, foods, and cosmetics.
Chromatoprobe -
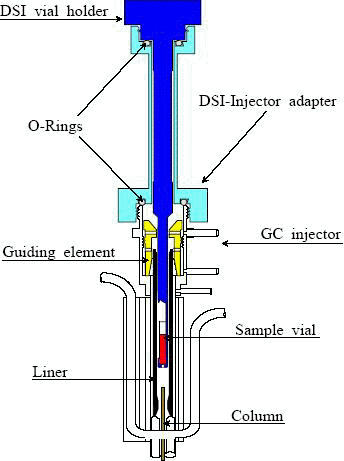
This piece of technology give us the ability to perform the analysis on the non-volatile sample. There is no need for sample preparation since we can put the hair, leaf etc. inside the little sample vial (you can see it in the picture, colored in red) and it stays there. Only the volatiles (and semi-volatiles) are extracted thermally from the vial. Notice that the tip of the chromatoprobe, with the sample vial, is inserted directly into the GC liner. The sample vial is disposed of after use.
"Each analysis begins with gentle solvent vaporization (if exists), preferably at a relatively low injector temperature, followed by brief heating of the injector to the desired temperature required for achieving intra-injector thermal extraction and sample compound vaporization. The sample semi-volatile compounds are focused (collected) on the early portion of the separation column and are GC analyzed as usual."
Our laboratory chromatoprobe site
The chromatoprobe is a commercial product that was developed at our laboratory in Tel Aviv university. you can read about it in Aviv Analytical's Website.
Atomic-Spectroscopy + ICP-MS back up back to Tal's main page
Well... it seems you have some trouble with the subject. I will try to clarify some things.
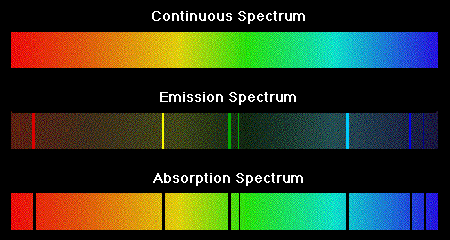
FAAS - Flame Atomic Absorption Spectroscopy (sometimes you will see AAF or other acronyms but AA always refers to Atomic Absorption).
We have the Hallow Cathode Lamp that produces spectral emissions of the elements in it (all of them ... if there are four elements all of them will give spectral emission lines). There will be also emissions of other species that are present in the plasma.
The produced light passes through the atomized sample (the light passes through the flame) and we get the absorption spectra (comparing the intensity of the light without the sample (blank) with the intensity with the sample).
One disadvantage of this technology is that we usually have to change lamps when we change the element we look for. This is bad news when we don't have the lamp we need, or we want to get information on several elements in the same sample thus having to run the sample several times changing the lamps in between.
FAES - when AE stands for Atomic Emission
In this method of operation we do not need the Hallow Cathode Lamp at all (we turn it off).
The flame itself thermally excites the electrons of the atoms it produces. When the electrons return to their unexcited orbitals they emit photons and produce an emission spectra (You saw one in the 'Spectrum of the Hydrogen atom' experiment in the Physical Chemistry lab).
This method is selective, since the flame's temperature will produce strong emission lines only to those elements that can be excited easily - the same elements with a low Ionization energy. This is why we will get a good emission spectrum out of Alkali metals.
For the reason above we can say that while AE gives us multiple elemental analysis, it cannot be applied to all elements.
Another thing about this method is that the flame must be kept in constant temperature, since minor differences in temperature will yield big differences in emission strength (since the temperature is in the exponent of the formula that determines the emission strength).
ICP-AE - Atomic Emission with a different method for atomization and excitation (instead of a flame) of the sample: Inductively coupled plasma.
The temperature inside the plasma is very high, therefore it successfully excites electrons and produces strong emission spectra from many elements (not only those with low ionization energy).
~ Universal.
ICP-MS - is an MS that analyzes the plasma that the ICP produces. We can say it is an elemental MS, since it will record the masses of the elements in the plasma.
It doesn't have anything to do with atomic spectroscopy of any sort.
Direct Insertion Probe back up back to Tal's main page
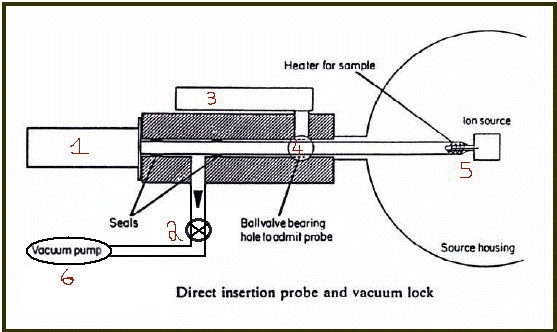
I will specify the correct operation order of this instrument:
a) Pull the handle (1) until the sample vial (5) passes the ball valve (4) but no further!
b) Close the ball valve (4) by using it's handle (3) so that the source housing (the chamber to the left) is isolated (can maintain vacuum).
c) Close the valve (2) to isolate the pump from the system (so that it will not be exposed to the atmosphere later).
d) Keep pulling the handle (1) until the sample vial passes the seals and exits completely.
e) Place a suitable sample in the sample vial.
f) Push the pole back inside using the handle (1) until is passes the seals.
g) Open valve (2) and allow it to pump the small chamber that ends in the ball (4).
h) Open the ball valve (4) using the lever (3).
i) push the handle (1) all the way so the sample reaches the ion source.
Questions and Answers back up back to Tal's main page
Q: How can we determine the concentration of two different compounds, using an HPLC, if their retention time is similar and they are not properly separated?
A: Since this questions is taken from one of the exercises I will only give you an overview and guide you to one possible answer :
Since the compounds are coeluting (they elute together) we need more information in order to quantify them. The additional information comes from the DAD (Diode Array Detector) in form of a multiple wavelength absorption spectrum.
If each of the compounds absorbs light in different wavelength we have no problem, we just inject each compound in a known amount and compare their absorption integral with that of the sample.
If both compounds absorbs light in similar wavelengths we have a bigger problem.
You will have to think how to use the fact that you can get (for the same analysis) many different chromatograms - taking them at different wavelength (this concept should be clear to you, if not please come to me and I will explain). You should remember Beer's Law and remember that the total absorption is the sum of all different absorptions.
Q: Regarding question 2a in exercise 3.
A: Since this questions is taken from one of the exercises I will only give you an overview and guide you to one possible answer :
The problem can be solved regarding two compounds coming out at almost the same time but without any column retention, this approach will give an upper limit to the value of the length.
you can calculate their width using the known formula (I gave it to you in class), calculate how much time (length dependent) will be needed to pass the optical path, and derive the length from an educated constraint placed on that equation.
Q: What are the advantages of a syringe (מזרק) pump over a piston (בוכנה) pump?
A: A syringe pump is much more accurate, and it produces a flow without pulsations (little rising and lowering of the pressure) so the flow is continuous. It is however limited by the amount of liquid it can run.
Q: What is the difference between an absorption detector and a fluorescence detector in LC?
A: To measure Absorption is the measure the lowering of light intensity by sample molecules. To measure Fluorescence is to measure the light emitted from sample molecules that where excited by light.
The Absorption detector simply collects the light passing through the sample and compares it's intensity with that of the light passing through the mobile-phase alone.
The Fluorescence detector is detecting light emitted from molecules in the sample, and since we don't want it to detect also the light that enters the sample (the light entering the sample is absorbed by compounds and then emitted back in all directions, either in the original wavelength, or in a different series or wavelength in case of fluorescence) we place the photomultiplier at 90º to the entering light path. since the fluorescence spectra is at different wavelengths than the absorbed light we also place a filter to screen out the wavelengths of the light that enters.
The sensitivity of the fluorescence detector depends only on the intensity of the light that we pass through the sample, and there is no upper limit to it, therefore there are VERY sensitive fluorescence detectors that use lasers for excitation.
Q: What is the difference between 'Photodiode array' and DAD?
A: There is no difference... they are the same (different companies call it by different names).
Q: Can PFPD recognize compounds such as NO ?
A: Yes, in PFPD (unlike NPD) there is no need for carbons to be present near the Nitrogen atoms.
Q: PB-LCMS spectrum, is it similar to a GC-MS spectrum (of the same molecule)?
A: Since the ion source is a EI ion source in both cases, and in both cases the sample molecules are heated, the spectrum will be very similar.
Q: Can't PFPD detect only Sulfur or Phosphorous like FPD?
A: PFPD can detect Carbons, Nitrogen, Sulfur and Phosphorous. You might want to take a look at the PFPD web-site.
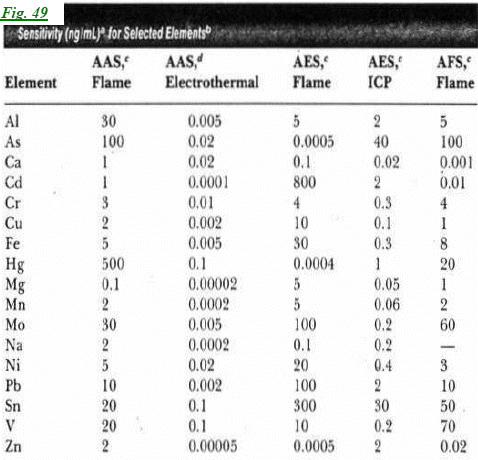
Q: We Wanted to know when to use FAE and when FAA (besides when there are more than 4 elements).
A: When we want to detect elements with low ionization energy we do not need a lamp at all. Actually, when we turn the lamp off, our equipment can still detect many elements (in the AE mode). Even if there are only two elements (less then four), we might not have a lamp with both of them inside it (one might be on one lamp and the other on a second lamp ... we cannot modify the lamps).
Another issue is the selectivity of the AE method. Since it applies only to elements with low-ionization energy, it can help us detect these elements in matrices (mixtures) even if they contain many other elements.
You might want to look at figure 49 in the booklet we gave you, it will show you when AA is more selective.
Q: We understood that GC-MS is one of the sampling methods in MS, but it seems that it's more than that, from the questions in some of the test. Can you clarify it?
A: GC is not a sampling method, it is a separation technology that can be attached to a MS. When we want to separate compounds in a mixture, and when these compounds are volatile enough, we can use the GC to separate them prior to the detection/identification. In most cases, the separation step is crucial for the identification on the analytes.
Q: In the MS-MS method, what does time-analysis means?
A: The idea of a MS-MS in time regards one of the abilities of the Ion-Trap. In a triple-quad MS, we can do MS-MS 'on the fly' by allowing only ions of a selected mass to pass the first quad, breaking them by causing collisions in the second quad, and then scanning the fragments using the third quad.
In Ion-Trap, we do all MS-MS steps (creating ions, isolation of a desired ion, breaking of that ion and analyzing it's fragments) in one chamber, each step occurring in a different time, not in a different place.
How to Study for the Final Exam - Tips back up back to Tal's main page
1) Sketches
In almost every exam the students are asked to draw a sketch of one of the technologies studied, and to specify the names of it's parts (sometimes there will be a requirement to explain the function of each part). This is a "Present" question in my opinion, since it is not hard to learn the sketches when you know how:
a) You must understand the use of the technology, and the function of it's different parts. For example: knowing that you can control the flow-rate of the carrier gas in the GC, you must know there should be a way to measure that flow, thus you will not forget the flow-meter part. Knowing that the PID detector needs optical isolation, you know to make the little chimney curved, and not strait.
b) Make a list of all drawings and go over that list at least three times : The first time you should mark a V for copying the sketch (drawing it while looking at what Aviv draw in the class), the second time mark a second V if you can draw it correctly without looking at the original and an X if you make a mistake (write down the mistake). The third time should give you the opportunity to correct your previous mistakes and make sure you still remember how to draw all the drawings.
2) Detector choices
Some of the questions are usually like the questions you received in your homework. If you encounter a question like "Choose a detector for the GC analysis of the hearth-medicine Lidocaine in human urine" and you do not know the composition of Lidocaine, you have a problem unless you know some general information about molecules that have the same usage. You should remember for example that most medicines have Nitrogen in them thus NPD might be a good choice.
Make a list of some general rules like that, and a list for the usages of every detector [NPD, as Aviv probably specified is used for drugs (medicine included of-course) {due to N} and pesticides {do to P}].
Make sure you memorize that list.
more will come (perhaps)...