 Question 04/05
Question 04/05
LINEAR MOLECULES
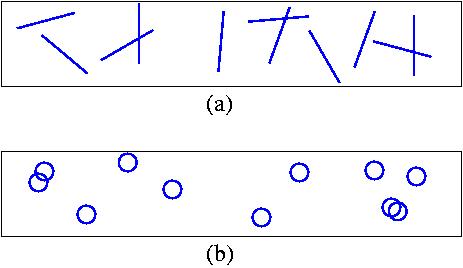
Consider a two-dimensional gas of molecules inside a rectangular
box of small width d and very large length.
If the box is filled by point-like molecules with density n,
then the pressure is p=nkBT.
(kB is the Boltzmann constant, and
T is the temperature.) If the point-like molecules
are replaced by thin linear molecules of length L, then
the pressure will slightly increase.
(a) Consider a case when L
is smaller (but not much smaller) than d,
and the molecules do not interact with each other, but they
cannot penetrate the walls of the box. (See picture (a).)
Calculate the exact pressure in the box.
(b) Consider a case when the molecules of lengths L
form rigid circles of diameter L/{pi}. (See picture (b).)
Calculate the exact pressure in the box.
(c) Note that your answers to (a) and (b) produced corrections
to the leading term (nkBT) that are simply
related to each other. What is the reason for that simple
relation?
 Back to "front page"
Back to "front page"
 Question 04/05
Question 04/05 Question 04/05
Question 04/05
 Back to "front page"
Back to "front page"