ChromatoProbe and SnifProbe
Sample Introduction Devices for Mass Spectrometry Sampling and GC and GC-MS Analysis
Aviv Amirav, Hongwu Jing, Alexander Gordin and Shai Dagan (June 2022)
The Concept and Summary of Features
We have designed, built and extensively tested a Direct Sample Introduction (DSI) device (USA and Japan patents [1]) that enables new sampling methods for GC and GC-MS. The DSI was licensed to and available from 1997 by Varian as the ChromatoProbe and later on from Bruker who acquired the Varian lab GC and quadrupole GC-MS business. It is now available by Agilent who licensed and introduced its own DSI under the name Thermal Separation Probe (TSP) (Agilent TSP). It is also available by FLIR under the name PSI Probe, by GL Science under the name DMI (automated DSI) and from Aviv Analytical (Aviv Analytical's ChromatoProbe Website) for mounting on the Agilent 6890 and 7890 GCs and 5973/5/7, 7000 and 7250 GC-MS systems. Over 1700 ChromatoProbes are currently in use worldwide and over one hundred and ten peer review publications were published (updated to 2011) on its various applications as listed at the bottom of this page. This DSI device serves for three different major applications, each with several advantages. As a result, the DSI (ChromatoProbe) is like three devices in a one product hardware:
A. Sample Introduction for Mass Spectrometry -The Cost Effective and Best MS Probe.
The ChromatoProbe device, followed by a short (1 m) capillary column, effectively transforms a conventional GC injector in a GC-MS system (preferably a second GC injector in a GC-MS) into a cost effective alternative to the standard direct insertion probe [2], with the following advantages:
 1.
Enables all the traditional functions of an MS probe, including
introducing thermally labile and polar compounds as well as high-mass
MS calibration and tune and other compounds.
1.
Enables all the traditional functions of an MS probe, including
introducing thermally labile and polar compounds as well as high-mass
MS calibration and tune and other compounds.
 2. Ideal
for developing and optimizing MS-MS and MS-MS-MS
methods.
2. Ideal
for developing and optimizing MS-MS and MS-MS-MS
methods.
 3. Low cost.
3. Low cost.
 4.
Faster and easier operation and interchange from
ChromatoProbe to GC-MS.
4.
Faster and easier operation and interchange from
ChromatoProbe to GC-MS.
 5. The
ChromatoProbe employs relatively big vials (1.9-2.4 mm I.D.) and as a result it
is much easier to use and introduce samples with it in comparison with standard
probe (0.6-0.8 mm vials I.D.). In addition, it uniquely accepts solution samples of solids/powders that are easier to prepare.
5. The
ChromatoProbe employs relatively big vials (1.9-2.4 mm I.D.) and as a result it
is much easier to use and introduce samples with it in comparison with standard
probe (0.6-0.8 mm vials I.D.). In addition, it uniquely accepts solution samples of solids/powders that are easier to prepare.
 6. No need for
changing ion source, no need for breaking vacuum, no need for
retuning the MS.
6. No need for
changing ion source, no need for breaking vacuum, no need for
retuning the MS.
 7.
Inherently immune against leaks and thus can be operated by untrained
personnel and students.
7.
Inherently immune against leaks and thus can be operated by untrained
personnel and students.
 8. Can
serve as a micro chemistry reactor. Enables liquid/solid CI agent
introduction, heavy water for D/H exchange, in vial
derivatization and limited temperature pyrolysis.
8. Can
serve as a micro chemistry reactor. Enables liquid/solid CI agent
introduction, heavy water for D/H exchange, in vial
derivatization and limited temperature pyrolysis.
 9. Field
adaptation. The ChromatoProbe
can be a true option for an easy future field installation.
9. Field
adaptation. The ChromatoProbe
can be a true option for an easy future field installation.
 10.
Dirty sample and SnifProbe chromatographic analysis capabilities as
described below.
10.
Dirty sample and SnifProbe chromatographic analysis capabilities as
described below.
B. Extract-Free Dirty Sample Introduction for GC and GC-MS Analysis.
Sampling is performed in a small vial that retains the harmful and non-volatile matrix residue of real world samples and thus eliminates the need for extraction or further sample clean-up [2-5, 13, 17-23]. The sample vial is disposed of after use. Each analysis begins with gentle solvent vaporization (if exists), preferably at a relatively low injector temperature, followed by brief heating of the injector to the desired temperature required for achieving intra-injector thermal extraction and sample compound vaporization. The sample semi-volatile compounds are focused (collected) on the early portion of the separation column and are GC analyzed as usual. This method was extensively tested by us in the analysis of drugs in raw urine and hair as well as pesticides in blended food items and in soil, and was found to have the following advantages:
 1. Lower
cost of analysis due to reduction or elimination
of sample preparation.
1. Lower
cost of analysis due to reduction or elimination
of sample preparation.
 2. Complex
small solid or sludge samples such as bacteria, tissue, gland, hair,
blood, urine, crude oil, soil and blended food items can be analyzed.
2. Complex
small solid or sludge samples such as bacteria, tissue, gland, hair,
blood, urine, crude oil, soil and blended food items can be analyzed.
 3.
Efficient thermal extraction. The excellent GC integrity (best among all thermal
desorption systems) enables high
and reproducible thermal extraction recovery that can potentially be
higher and more uniform than that of standard solution extraction (or
that of external thermal extraction).
3.
Efficient thermal extraction. The excellent GC integrity (best among all thermal
desorption systems) enables high
and reproducible thermal extraction recovery that can potentially be
higher and more uniform than that of standard solution extraction (or
that of external thermal extraction).
 4. Smaller
sample size. A very small sample can be used and the extraction solvent
impurities is eliminated.
4. Smaller
sample size. A very small sample can be used and the extraction solvent
impurities is eliminated.
 5. Higher
sensitivity is enabled through the sampling of a large volume
concentrated extracts.
5. Higher
sensitivity is enabled through the sampling of a large volume
concentrated extracts.
 6. Faster
chromatographic analysis is achieved as the less volatile matrix
compounds are retained in the vial, enabling a lower upper GC oven
temperature.
6. Faster
chromatographic analysis is achieved as the less volatile matrix
compounds are retained in the vial, enabling a lower upper GC oven
temperature.
 7. Triple
purpose device. The same ChromatoProbe
device also serves as an MS probe as above in GC-MS and as a
SnifProbe as described
below.
7. Triple
purpose device. The same ChromatoProbe
device also serves as an MS probe as above in GC-MS and as a
SnifProbe as described
below.
C. SnifProbe Gas Sampling Method and Device
SnifProbe is a
major additional capability of the ChromatoProbe that extends its use for
airborne compounds analysis. It is described in details in references 9 and 41.
SnifProbe is based on
the use of 15 mm short pieces of standard 0.53 mm ID capillary or PLOT
column or silicone rubber (PDMS membrane) tube loaded vials for sampling air born, head space, aroma or air pollution
samples. A miniaturized frit-bottomed packed micro vial named
MicroSPE was also
prepared and served for the sampling of solvent vapors and gases as
well as liquid water.
Thus, SnifProbe extends the ChromatoProbe
range of samples to include gas phase sampling. The short (15 mm)
column is inserted into the SnifProbe
easy-insertion port and the SnifProbe
is located or aimed at the sample environment. A miniature pump is
operated for pumping 20-60 ml/min (typically 50 ml/min) of air sample
through the
sample collection short piece of column. After a pre-selected several
seconds
of pumping, the short column is removed from the
SnifProbe with a tweezers and placed inside a
ChromatoProbe
glass vial having a 0.5 mm hole at its bottom. The
ChromatoProbe
sample holder with its glass vial and sample in the short column are
introduced into the GC injector as usual. The sample is then quickly
and efficiently vaporized from the short sample column and is
transferred to the analytical column for conventional GC and or GC-MS
analysis.
SnifProbe enables
many of the manual SPME, air bags and Tenax tube applications with a
few advantages, and is ideal for out of the laboratory sample
collection, head space and air born sample analysis:
 1.
SnifProbe brings the
field and process into the laboratory. SnifProbe can be operated in
the field or at the process, and the sample columns can be plugged,
placed
in a small plastic bag, marked and brought to the laboratory for
analysis
with the full power of lab GC/GC-MS.
1.
SnifProbe brings the
field and process into the laboratory. SnifProbe can be operated in
the field or at the process, and the sample columns can be plugged,
placed
in a small plastic bag, marked and brought to the laboratory for
analysis
with the full power of lab GC/GC-MS.
 2. Thermally
labile and semi-volatile compounds such as explosives and CWA can be
collected since the trapping column is easier to desorb than Tenax
tubes.
2. Thermally
labile and semi-volatile compounds such as explosives and CWA can be
collected since the trapping column is easier to desorb than Tenax
tubes.
 3. Compact.
SnifProbe has small size
and its miniature sample columns, silicone rubber loaded vials or
MicroSPE vials are easy to transport
and can be sealed for many weeks.
3. Compact.
SnifProbe has small size
and its miniature sample columns, silicone rubber loaded vials or
MicroSPE vials are easy to transport
and can be sealed for many weeks.
 4. Fast sampling
and GC analysis. Large sample volume is quickly probed and fast GC
analysis (under 1 minute) is enabled with only a small split ratio of
2:1.
4. Fast sampling
and GC analysis. Large sample volume is quickly probed and fast GC
analysis (under 1 minute) is enabled with only a small split ratio of
2:1.
 5. Uniform
response. The narrow column diameter ensures that all molecules are
adsorbed. If the sampling time is limited, all the sample compounds are
retained.
5. Uniform
response. The narrow column diameter ensures that all molecules are
adsorbed. If the sampling time is limited, all the sample compounds are
retained.
 6. Built-in
external protection. Since the adsorption layer is internal, the short
sample column can be introduced into difficult locations while lightly
touching the matrix. The sniffing column can also be fully covered
except its opening.
6. Built-in
external protection. Since the adsorption layer is internal, the short
sample column can be introduced into difficult locations while lightly
touching the matrix. The sniffing column can also be fully covered
except its opening.
 7. High
sensitivity. Since SnifProbe
is based on active air pumping, a large
sample volume can be quickly probed for increased sensitivity.
7. High
sensitivity. Since SnifProbe
is based on active air pumping, a large
sample volume can be quickly probed for increased sensitivity.
 8. Dirty/reactive
sample analysis capability. Since the sample short columns cost very
little they can be employed for dirty or reactive sample analysis and
be disposed of after the analysis. (cigarette smoke for example)
8. Dirty/reactive
sample analysis capability. Since the sample short columns cost very
little they can be employed for dirty or reactive sample analysis and
be disposed of after the analysis. (cigarette smoke for example)
 9. A broad range
of sample column adsorption films and materials is available for sample
collection optimization. From thin dimethylsilicone through thick PLOT
column films and including the full range of packed
column adsorption materials.
9. A broad range
of sample column adsorption films and materials is available for sample
collection optimization. From thin dimethylsilicone through thick PLOT
column films and including the full range of packed
column adsorption materials.
 10. Very cost
effective. SnifProbe is
coming with the full power of ChromatoProbe of extract free dirty
sample introduction and its use as an MS probe.
10. Very cost
effective. SnifProbe is
coming with the full power of ChromatoProbe of extract free dirty
sample introduction and its use as an MS probe.
 11. Field installation.
SnifProbe
can
be operated with a DSI/ChromatoProbe that can be field installed on
any GC standard split splitless injector and does not requite a PTV for
its sampling.
11. Field installation.
SnifProbe
can
be operated with a DSI/ChromatoProbe that can be field installed on
any GC standard split splitless injector and does not requite a PTV for
its sampling.
SnifProbe is available exclusively by Aviv Analytical (Aviv Analytical SnifProbe).

Technical Description
The DSI
is based on sample introduction into the GC injector in a small
disposable test tube (sample micro vial) that is commercially available
for standard probes for their insertion into the ion sources of mass
spectrometers
(Varian, Walnut Creek CA, Scientific Instrument Services, Ringoes NJ).
Most of the standard temperature programmable GC injectors can be
adapted
to accept a DSI
device.
In 1997 the DSI
became
commercially available by Varian (named ChromatoProbe) and is compatible with Varians’
1078 and 1079 injectors. Thus, in this page will shall name the
DSI as
ChromatoProbe
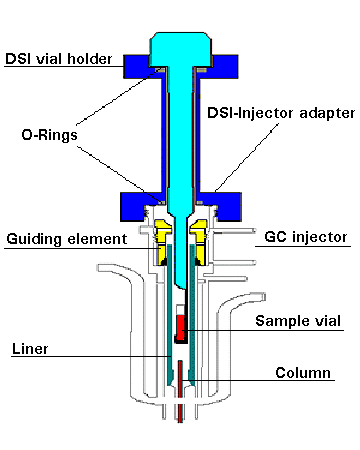
The structure and components of the ChromatoProbe,
mounted on the Varian 1079 injector is presented in Figure 1. The
original septum and its seat were removed and the septum clamp was
replaced with a guiding element unit. This unit serves for sealing on
the injector body and guiding the sample holder that is inserted
through the injector adapter. The liner is the standard large volume
liner (3.4 mm ID), which is inverted so that its open end is positioned
upward to accept the sample holder.
The sample holder was designed for glass test tubes (mirco vials) with
2.5 mm OD, 1.9 mm ID and 6 to 18 mm in length. The standard length is
15
mm and its volume is 40 microLiters. The diameter size restriction
depends
on the injector liner used. Quantitative liquid sample introduction
into
the test tube can be performed with a standard 10 microLiter GC syringe
(Hamilton 1701N for instance), while the test tube is held with a
tweezers
(or gloved hand). Solid and powder samples can be introduced with a
standard
Pasteur pipette directly into the vial. The sample container holder is
fabricated
from stainless steel (such as the 316 or Inconel), then coated, either
with Silcosteel (Restek), or with a high temperature lacquer for
surface passivation. The Varian and Aviv Analytical
ChromatoProbe are provided with Silcosteel passivation.

Direct Sample Introduction for Mass Spectrometry Studies - The Cost Effective Probe
The device shown in Figure 1 effectively transforms a conventional
GC injector into a direct sample introduction (DSI) device (Probe) for the continuous
delivery
of sample compounds into the MS. The ChromatoProbe/DSI is a low cost and simple device.
Even the cost of an additional second GC injector added to the
ChromatoProbe
device is lower than that of an air lock chamber, by-pass pumping
system and
rotary pump, and temperature controls involved with standard direct
sampling
probes.
The GC injector temperature, flow rate and split ratio effectively
control the vaporization rate and MS signal. For the application of
mass spectrometry direct sampling, it is recommended to use a short
column (2 meter, 100 micron ID, no or thin (0.1 micron) film thickness)
heated to a relatively high temperature that serves as a fast transfer
line. The column temperature should be high enough so that the sampled
compounds are not retained on the column. During ChromatoProbe
operation, the sample is loaded
inside the test tube and the flow rate is increased to a typical flow
of
1 ml/min in the microbore short column, resulting in a one second
response
time. The preferred configuration is to use a dual-injector,
dual-column system. One of the columns is the short one for MS probing,
and the other is the usual GC separation (analytical) column. Both
columns are introduced into the MS via a double-hole ferrule (provided
by Varian). The carrier gas
flow rate through the short column can be reduced below 0.2 ml/min when
not
in use, so that it does not affect the conventional GC-MS performance.
The
analytical column flow rate can be reduced to below 0.4 ml/min during
probe
sampling.
The ChromatoProbe, used as a direct sampling probe can serve for several applications:
 1. Conventional applications of a
direct sampling probe. It enables the sampling of solids and powders
including high boilers and thermally labile compounds. The fast
response time, and the inert inlet and transfer line, provide good
response for these sensitive compounds.
1. Conventional applications of a
direct sampling probe. It enables the sampling of solids and powders
including high boilers and thermally labile compounds. The fast
response time, and the inert inlet and transfer line, provide good
response for these sensitive compounds.
 2. MS-MS optimization. The
ChromatoProbe is
an excellent tool for MS-MS method development and optimization. It
also enables MSn studies. The
ChromatoProbe
should be therefore an inseparable part of any package containing MS-MS.
2. MS-MS optimization. The
ChromatoProbe is
an excellent tool for MS-MS method development and optimization. It
also enables MSn studies. The
ChromatoProbe
should be therefore an inseparable part of any package containing MS-MS.
 3. Solution sampling. Unlike standard
probes, the ChromatoProbe
can accept solutions, where the solvent can be gently evaporated at 1
Atm, in the
injector, to the open split vent, prior to sampling the solute. Powders
can also be sampled with a syringe through their crude solvation.
3. Solution sampling. Unlike standard
probes, the ChromatoProbe
can accept solutions, where the solvent can be gently evaporated at 1
Atm, in the
injector, to the open split vent, prior to sampling the solute. Powders
can also be sampled with a syringe through their crude solvation.
 4. Liquid Agents Introduction. Direct
sampling through the GC injector can serve for on-line, separately
optimized, reagent introduction into
the MS ion source during GC-MS analysis. This procedure may be used in
several applications including:
4. Liquid Agents Introduction. Direct
sampling through the GC injector can serve for on-line, separately
optimized, reagent introduction into
the MS ion source during GC-MS analysis. This procedure may be used in
several applications including:
a) Introduction of a liquid (or solid) compound for serving as
a chemical ionization (CI) reagent, with tailored proton affinity.
b) Introduction of heavy water or deuterated methanol for
the exchange of labile hydrogen atoms with deuterium atoms in OH or NH
groups for their identification.
c) High-mass calibration compounds (such as PFK
(perfluorokerosene)) can be constantly introduced for on-line high
and/or accurate mass calibration.
 5. Micro-Chemistry Reactor. Direct
sampling through the GC injector enables the application of selective
chemical reactions such as oxidation that requires rough atmospheric
pressure, or solution derivatization (inside the micro test tube) [10].
The injector temperature programming enables
temperature programmed pyrolysis and material testing studies within
the
temperature limitations of the system (typically 450 C upper injector
temperature).
5. Micro-Chemistry Reactor. Direct
sampling through the GC injector enables the application of selective
chemical reactions such as oxidation that requires rough atmospheric
pressure, or solution derivatization (inside the micro test tube) [10].
The injector temperature programming enables
temperature programmed pyrolysis and material testing studies within
the
temperature limitations of the system (typically 450 C upper injector
temperature).
 6. MS-MS Analysis. The
ChromatoProbe
enables fast medium complexity sample analysis using MS-MS for sample
separation and quantitative determination. For very complex mixtures
the ChromatoProbe
enables extract free sampling (as explained below) followed by GC-MS
and/or GC-MS-MS analysis [13, 19].
6. MS-MS Analysis. The
ChromatoProbe
enables fast medium complexity sample analysis using MS-MS for sample
separation and quantitative determination. For very complex mixtures
the ChromatoProbe
enables extract free sampling (as explained below) followed by GC-MS
and/or GC-MS-MS analysis [13, 19].

Suggestions for optimized use of the ChromatoProbe for MS studies:
1. In order to install the preferred configuration of two columns, one for the ChromatoProbe and the other for conventional GC applications, please use a two-hole ferrule (supplied) for the coupling of the two columns together into the MS.
2. If an autosampler is coupled to the GC, the ChromatoProbe device should be located at the front injector, and the autosampler should be coupled to the rear injector.
3. In order to avoid the risk of inlet contamination, it is recommended to introduce solutions and not neat materials. The solvent has to be gently vaporized first at a temperature of 15-20 C above its boiling point (1 min), and then, the inlet has to be further heated to the proper sampling temperature. 5 microLiter of a solution of 2 mg/ml that desorb the sample compound at a rate of 10 ng/sec with split ratio 10:1 (1 ng/sec into the ion source) will produce an appropriate signal for more than 15 minutes.
4. The temperature of the inlet during sampling should be experimentally determined to be the minimum temperature that produces a sufficient signal. This will prevent inlet and ion source contamination as well as assure minimum thermal degradation of thermally labile compounds. (this is unlike with standard probes which require the control of the ion source temperature)
5. The inlet should preferably be operated in the split mode, while the split ratio has to be determined depending on the nature of the sample and the sensitivity required. This prevents MS overloading, reduces inlet contamination and minimizes inlet related dissociation.
6. During the vial holder insertion, the column should be kept at a low temperature (<100 C) to avoid the risk of column damage due to air penetration. Preset conditions of high flow / high pressure may further reduce the probability of air penetration. After inserting the vial and screwing back the vial holder, the temperature of the column should be elevated to a temperature, high enough to allow unretained, immediate elution of the analyte. This can be an automatic feature in the ChromatoProbe method.
7. When the analysis is finished, the vial has to be taken out and disposed of while the empty vial holder is screwed back. The GC inlet and column should be then heated for a short time (few minutes) until there is no trace of the sampled compound in the observed mass spectrum. During that time the split vent should be open. This step is essential only if high cleanliness is desirable.
8. If a GC analysis has to be performed soon after the ChromatoProbe analysis, one can cool down the ChromatoProbe injector, reduce the flow at the ChromatoProbe short column to a minimum of 0.2 ml/min (and thus reduce any ChromatoProbe trace signal, if exists), open its split flow and immediately start a GC run at the second, chromatographic column.
9. New probes should be sonicated with solvents prior to use. A lacquer-coated probe should be thermally cured after coating, following the manufacturer instructions (250 C in air, under a dirt cover, for 30 minutes). A Silcosteel coated probe should be baked at 250-280 C for a few hours while it is in the GC inlet, with carrier gas flowing, and split vent open. Silcosteel coating may be damaged at very high temperatures (over 250 C) in the presence of air.

Dirty Sample Introduction for GC and GC-MS Analysis
The ChromatoProbe
can serve as an introduction device for "dirty" untreated samples in
any form of liquid, solid, powder or slurry. These include:
a) Body fluids and other human matrices such as urine [2],
blood or plasma, hair [5], tissues and bacteria.
b) Food items such as blended fruits, vegetables and spices
[3-4,13, 16].
c) Environmental samples such as crude oil, asphalt, soil
or dust [15].
d) Other industrial matrices such as paint, glue and polymers
(desorption and/or pyrolysis).
The approach of using the ChromatoProbe for the gas
chromatographic analysis of dirty samples may eliminate, or
significantly reduce the sample preparation steps of extraction,
cleanup and pre-concentration, and allow in-vial chemical reactions
such as derivatization. The procedure of analyzing such samples with
the ChromatoProbe
involves loading the sample into the vial (in a quantitative way),
performing gentle solvent vaporization (if a solvent exists), analyte
thermal extraction at the inlet, and then continuing with the standard
GC analysis. The basic goal in developing a method for a specific
sample is to find conditions in which the compounds of interest are
selectively and efficiently thermally extracted, without scarifying the
liner and column cleanliness. The solvent and other unwanted volatile
materials might be vented through the split vent, and non volatile,
high boiling and labile matrix elements are left in the micro vial, to
be disposed of after the analysis. In Figure 2, we present
a suggested strategy of programming the GC parameters in order to
obtain
"extract free" DSI sampling of dirty samples.
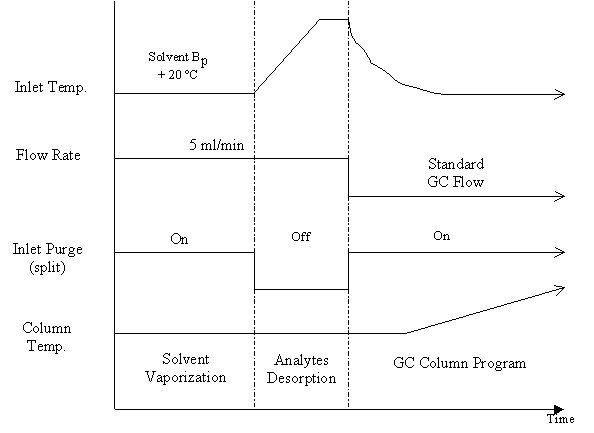
Figure 2 Time Programming Scheme of GC parameters for DSI sampling of "dirty" samples for GC or GC-MS analysis.
The scheme in Figure 2 is a splitless introduction of a liquid (or liquefied) dirty sample introduced with the ChromatoProbe device. If a solid sample is introduced one can skip the solvent vaporization step.
Solvent Vaporization: About 1 min is required for volatilization of up to 4 microLiter of solvent when the inlet is at 15-20 C above the solvent’s boiling point. The purge vent (split valve) should preferably be open at this time to speed-up the vaporization and minimize the amount of solvent and other volatile unwanted compounds entering the column. If the boiling point of the analyte is close to that of the solvent, the split valve should be closed in order to prevent any analyte loss during the solvent vaporization period. A purge flow of more than 20 ml/min is recommended. An introduction of a sample amount of 2-4 microLiter is recommended although up to 25 microliter quantity has been successfully tested. Special care has to be taken in order to avoid solvent splashing and contamination of the GC liner. If the sample contains a mixture of two or more solvents having different boiling temperatures, one can consider slow temperature programming of the inlet at this stage, in order to "gently" evaporate the solvent mixture.
Analyte Desorption: The purge valve is turned off in the extraction period in order to collect all the desorbed molecules. A flow rate of 5 ml/min or more is necessary to achieve efficient sweeping of the desorbed sample compounds. The maximum inlet temperature and hold time should be experimentally determined. The considerations leading to the choice of these parameters are efficient desorption of all the analytes on the one hand, but on the other hand, minimum decomposition and desorption of higher boiling and labile matrix elements that should be retained in the vial. For general pesticide and drug analysis, 250° C for 0.1-0.5 minutes was found to be satisfactory. In pyrolysis experiments (micro organisms, polymers) one should consider efficient decomposition and desorption of the pyrolysis products. The heating rate of the inlet depends on the thermolability of the analytes and matrix. A typical rate can be 150 C/min or more. Fast (forced) cooling of the inlet is essential in order to achieve more precise control over the desorption process as well as to prepare for the next analysis. Thus, a cooled (PTV) injector must be used with the ChromatoProbe.
GC program: After the thermal desorption step, the carrier gas flow rate is reduced to the normal value for optimized GC performance. The purge (split) valve is turned on in order to avoid any solvent peak tail. The oven is heated at the usual program. If volatile analytes (close to the solvent in their elution time) are to be analyzed, it is recommended to start the GC oven at a low temperature that enables column cryo-focusing of the volatile analytes. In some cases, one may need to wait at the column start temperature until all the solvent residue has eluted in order to improve the separation between the residual solvent and the analyte and hence obtain a proper GC peak of the volatile analyte. The upper GC temperature and/or wait period may be reduced since the non-volatile and high boiling matrix residue compounds are retained in the vial.
In Figure 3 below we demonstrate the application of the ChromatoProbe for the fast analysis of cocaine in a single hair of human drug user. For details please read reference 5
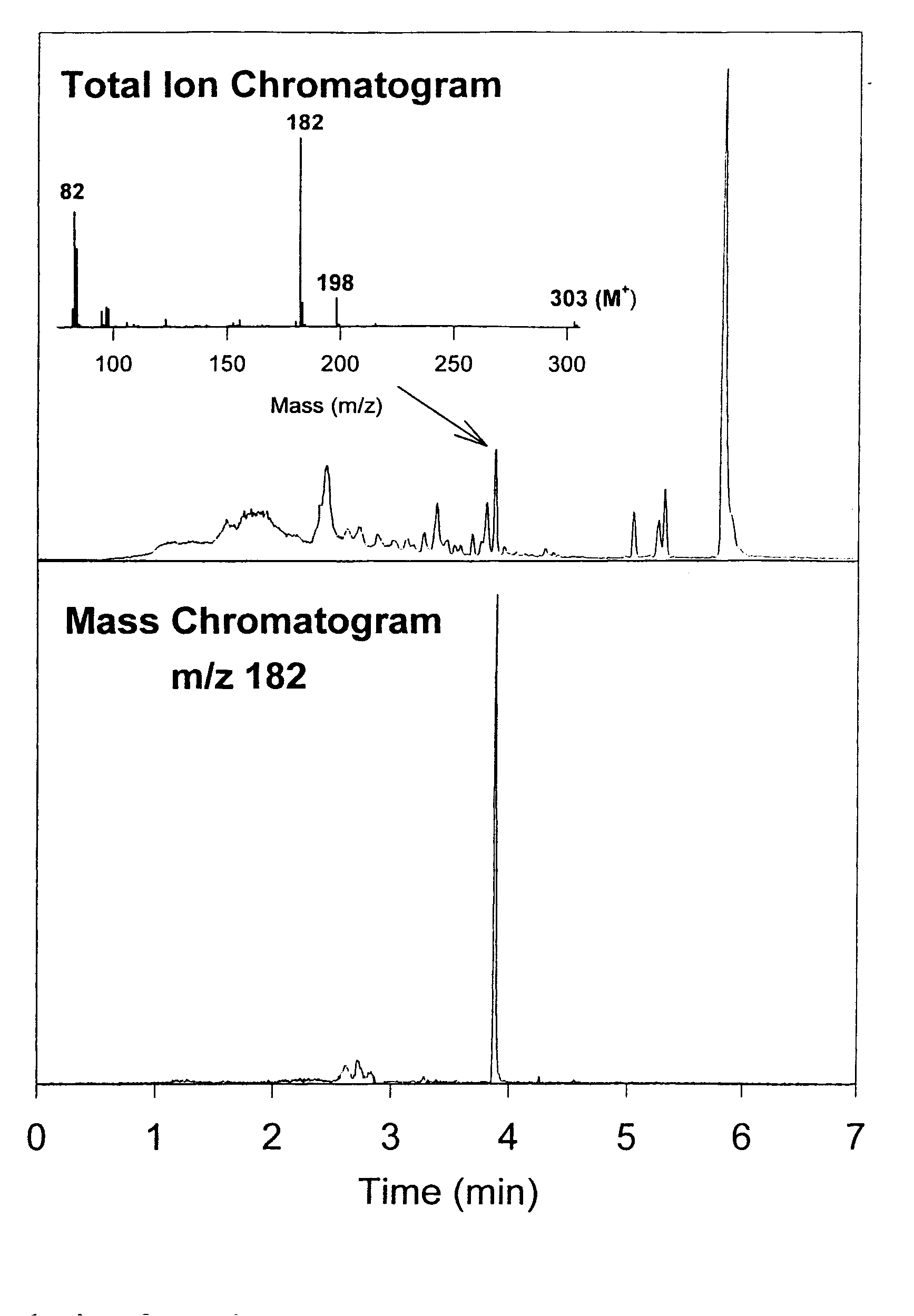

Additional Guidelines for the Application of the ChromatoProbe in the Chromatographic (GC and or GC-MS) Analysis of Dirty Samples
1. The sample micro-vial should be handled with tweezers only. The handling with powder free gloved hands can be tested. Similarly, the micro-vial holder should not be touched with hands to avoid fingerprints and dirt being included in the analysis.
2. Solid samples may be blended or dissolved for a more quantitative transfer, as well as for more efficient thermal extraction from the micro-vial.
3. The volume of a 15 mm long vial is ~40 microliters. Up to 25-30 micro liters can be loaded into that vial. Solvent evaporation can be achieved either in the GC inlet (if relatively volatile compounds are to be analyzed) or preferably outside the GC prior to sample introduction. The GC split gas flow can be used for such external evaporation. A special "tool" can help in minimizing the solvent vaporization time.
4. A pressure drop in the inlet occurs when the ChromatoProbe cap is opened during sample introduction and its reverse pressure build up can be too slow. Pressing "Activate" of the method and than "Activate now" and "Start" (in the Varian 3800 GC), immediately after screwing back the loaded vial holder, would enable fast pressure buildup. No such problem exists with constant pressure injectors that have no EFC. The current Varian CP 3800 GC has faster pressure build up and with it this remark can be ignored.
5. The initial column temperature should be low enough to trap the extracted volatile compounds. This is like in a conventional splitless injection with about 2.5 minutes injection time. For example, 50-80 C initial column temperature is desirable for pesticide analysis.
6. Note that the final GC oven upper temperature and time may be reduced compared to conventional GC analysis since the less volatile compounds are retained in the micro-vial. Typically, the upper GC temperature should be higher by no more than 50C from the upper injector thermal desorption temperature. This may save time and extend the column lifetime.
7. At the end of the analysis, please dispose of the sample micro-vial. Do not re-use micro-vials. The micro-vial holder should only be removed from the adapter after both the GC oven and injector were cooled and a carrier gas purge flow from the DSI device protects the column. Standard columns are fully air safe below 140C.
8. In addition to passivation of the micro-vial holder, analysis of thermally labile sample compounds can be facilitated with a short micro-vial (6 mm long), minimizing the interaction between the analyte and the vial and allowing extraction at a lower temperature. The standard 15 mm long micro-vials can be cut (like a column) to the desirable length. Handle the micro-vial carefully while cutting to avoid contamination. Such short vials can be ordered from SIS (Ringoes, NJ, USA). The split flow rate can be higher as well.
9. Test your method for thermal vaporization efficiency, reproducibility and long term stability before beginning routine analysis.
10. Dirty sample fast GC analysis with the ChromatoProbe can be explored and employed using the 2 meter microbore column supplied for probe sampling, while implementing a proper GC program for dirty sample analysis. The major problem with this short column is its limited sample capacity but it can help in the study of the principles of use of DSI for dirty sample analysis. Actually, this is similar to using the ChromatoProbe as a probe with a relatively cool initial GC temperature. A fast GC or GC-MS analysis can be achieved with fast GC oven temperature programming rate.

SnifProbe suggestions and recommendations:
The use of SnifProbe for gas analysis is the latest development that extends the capabilities of the ChromatoProbe and thus only preliminary remarks are mentioned here. Its use and applications is described in details in reference 9. SnifProbe, unlike ChromatoProbe, is not yet commercially available, but we very much hope it will be. We consider its use as relatively simple and encourage every one who performs gas analysis to explore it.
In Figure 4 below the SnifProbe structure and various components are outlined. For further details reference 9 is recommended for reading and can be provided upon request.
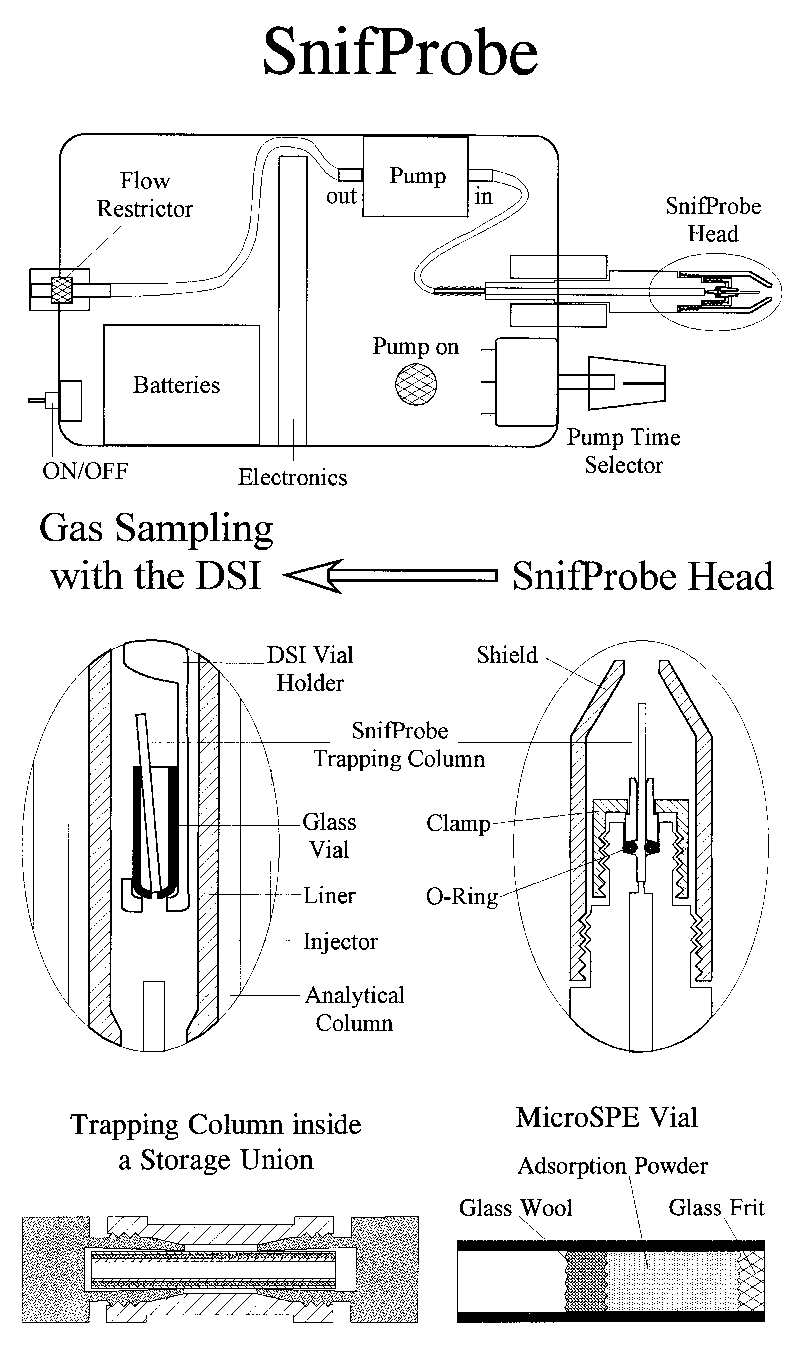
In order to effectively start working with SnifProbe please consider the following list of advises:
1. A miniature pump such as ASF model 3003 should be used for 6-60 ml/min air pumping. We found that a Mott 250 ml/min flow restrictor element at the input of the pump stabilizes its flow rate and reduces it to about 30 ml/min.
2. An SGE removable needle standard syringe such as model 10R can be modified at the machine shop so that its head will accept a 0.7 mm OD columns with two Parker number 1 Viton O-Rings like a Wilson seal.
3. The modified syringe needs to be connected with a Wilson seal into the air pump.
4. Please use a ChromatoProbe standard glass vial and make a 0.5 mm hole at its bottom. The hole should be small enough to prevent the sample column from falling down. The glass vial desirable length is 10 mm.
5. Please use gloves and cut a 0.53 mm ID column of your choice into several pieces with 15 mm length. The length should be reproducible. The preparation of MicroSPE is a little more demanding and may require our consultation.
6. For volatile compound analysis with boiling points in the range of 0-150 C please use the Chrompack CarbBOND PLOT column. For compounds in the 80-250 C boiling point range the Chrompack PoraBOND PLOT column seems ideal. For semivolatile compound in the 150-400 C boiling point range a standard Rt5 column with 5 micron film is ideal. For solvents and or permanent gases MicroSPE vials should preferably be used. A MolSieve PLOT column can be tested with a standard vial without a hole for the analysis of permanent gases.
7. Please wait at least 3 minutes after the DSI sample holder was removed from the GC injector to let it cool down to avoid its higher temperature from inducing undesirable vaporization. Alternatively and preferably please use the second vial holder that is at room temperature (provided with the ChromatoProbe kit).
8. The GC injector temperature can be in the range of 150-300 C for SnifProbe sample column desorption, depending on the application and the column used. Low injector temperature can be used with injector temperature programming but generally no injector temperature programming is necessary.
9. Please use tweezers for sample column handling and avoid touching it or making contact with a dirty surface.
10. For field analysis the use of a modified HPLC 1/16 inch Peek union as a storage device is recommended as shown in the SnifProbe figure
11. Every analysis should be tested and optimized first at the laboratory. The pumping time and way of ChromatoProbe sample container insertion should be reproducible for achieving reproducible results.

Demonstrations and Applications:
35 figures of demonstrations and applications are shown in the
ChromatoProbe and SnifProbe booklet that is
available on request. They were achieved using several
combinations of the ChromatoProbe
device with several GCs, GC detectors and MS systems. The technology of
supersonic molecular beam mass spectrometry (SMB-MS), including fast
GC-MS and unique ionization methods of electron ionization (EI) in SMB
and hyperthermal surface ionization (HSI), is reviewed in references
[6, 7]. HSI is implemented for the analysis of drugs in hair in
reference [5]. Detection with the pulsed flame photometric detector
(PFPD) is reviewed in references [3, 8]. The DSI-PFPD-MS combination is
reviewed in reference [4].
The issues that are addressed and demonstrated in
ChromatoProbe and
SnifProbe booklet are:
 1. MS probe sampling.
1. MS probe sampling.
 2. Thermal desorption efficiency and
characteristics in dirty sample analysis. In that part we demonstrate
the effect of flow rate and extraction temperature on the extraction
efficiency and selectivity.
2. Thermal desorption efficiency and
characteristics in dirty sample analysis. In that part we demonstrate
the effect of flow rate and extraction temperature on the extraction
efficiency and selectivity.
 3. Analysis of drugs in untreated raw
human urine.
3. Analysis of drugs in untreated raw
human urine.
 4. Fast analysis of drugs in an untreated
single human hair.
4. Fast analysis of drugs in an untreated
single human hair.
 5. Analysis of pesticides in fruits,
vegetables and spices.
5. Analysis of pesticides in fruits,
vegetables and spices.
 6. Sensitivity enhancement employing large
volume extract introduction.
6. Sensitivity enhancement employing large
volume extract introduction.
 7. Analysis of pesticides in soil - an
environmental sample.
7. Analysis of pesticides in soil - an
environmental sample.
 8.
ChromatoProbe-GC-PFPD-MS combination:
In the analysis of trace levels of pesticides in complex matrices,
detection capability and mass spectral library identification is often
hampered by the co-elution of matrix compounds. Simultaneous PFPD-MS
analysis
is performed with column effluent splitting between these two
detectors. The resulting PFPD chromatograms are always much simpler due
to its selectivity and exhibit better sensitivity than that of the MS.
Accordingly, the PFPD chromatogram serves as a marker. At the PFPD
given exact elution time,
the resulting mass spectra are examined for unique mass peaks, and
precise
background subtraction is performed for improved library
identification.
Moreover, the information on the presence of P and/or S atoms in the
analyte
can be incorporated in a constrained library search (such as the NIST
sequential search), resulting in lower identification levels. Major
anticipated applications of the PFPD-MS approach are pesticide
analysis, CWA detection and identification and unknown sulfur compound
identification in complex matrices. The combination of DSI sampling
with PFPD-MS analysis is an integrated approach for truly fast
screening and confirmation. The PFPD-MS method is further explained in
detail in reference [4].
8.
ChromatoProbe-GC-PFPD-MS combination:
In the analysis of trace levels of pesticides in complex matrices,
detection capability and mass spectral library identification is often
hampered by the co-elution of matrix compounds. Simultaneous PFPD-MS
analysis
is performed with column effluent splitting between these two
detectors. The resulting PFPD chromatograms are always much simpler due
to its selectivity and exhibit better sensitivity than that of the MS.
Accordingly, the PFPD chromatogram serves as a marker. At the PFPD
given exact elution time,
the resulting mass spectra are examined for unique mass peaks, and
precise
background subtraction is performed for improved library
identification.
Moreover, the information on the presence of P and/or S atoms in the
analyte
can be incorporated in a constrained library search (such as the NIST
sequential search), resulting in lower identification levels. Major
anticipated applications of the PFPD-MS approach are pesticide
analysis, CWA detection and identification and unknown sulfur compound
identification in complex matrices. The combination of DSI sampling
with PFPD-MS analysis is an integrated approach for truly fast
screening and confirmation. The PFPD-MS method is further explained in
detail in reference [4].
 9.
SnifProbe applications including the analysis of BTX in
air, cigarette smoke, mercaptanes in domestic cooking gas, ethanol in
human breath after beer drinking, trace level chemical warfare agent
simulants in air, coffee aroma and perfume in air.
9.
SnifProbe applications including the analysis of BTX in
air, cigarette smoke, mercaptanes in domestic cooking gas, ethanol in
human breath after beer drinking, trace level chemical warfare agent
simulants in air, coffee aroma and perfume in air.
 10.
SnifProbe
with MicroSPE
sample trap applications including the analysis of solvent vapors, SO2
in air and BTX in water.
10.
SnifProbe
with MicroSPE
sample trap applications including the analysis of solvent vapors, SO2
in air and BTX in water.
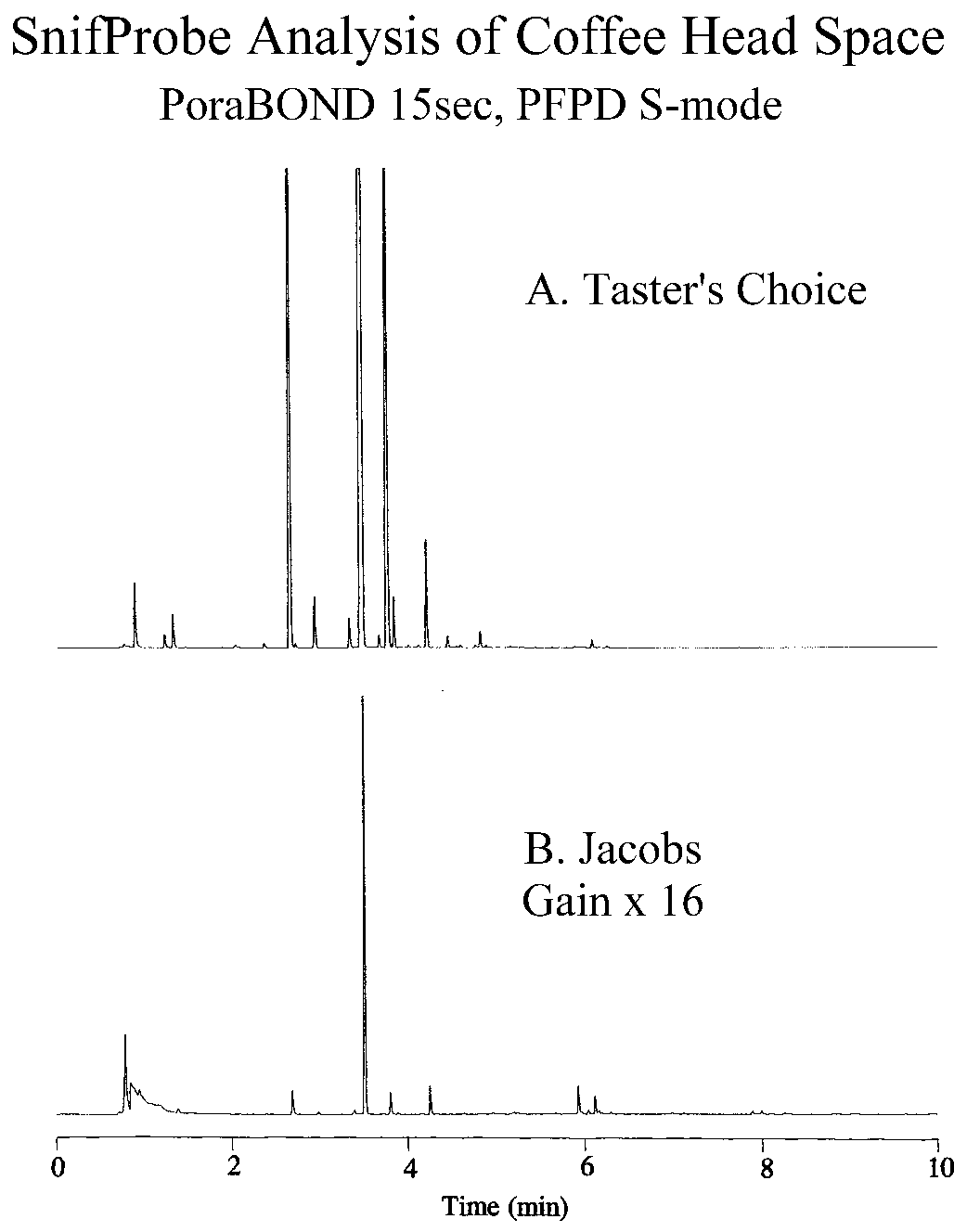
In Figure 5 below, the use of SnifProbe for the sampling and analysis of coffee aroma is demonstrated. A GC with PFPD system was used for the selective detection of sulfur compounds in the aroma of instant coffee. Note the much greater abundance of sulfur compounds in the aroma of the Taster's Choice brand over that of the Jacobs brand.

References
( marks recommended for reading).
marks recommended for reading).
1. A. Amirav and S. Dagan , "Method and Device for the Introduction of a Sample into a Gas Chromatograph." U.S. patent No 5686656 and Japan patent No 3191147.
 2. A. Amirav and S. Dagan, "A Direct Sample
Introduction Device for Mass Spectrometry Studies and GC-MS Analysis",
Europ. Mass.
Spectrom. 3, 105-111 (1997). (Original DSI/ChromatoProbe paper)
2. A. Amirav and S. Dagan, "A Direct Sample
Introduction Device for Mass Spectrometry Studies and GC-MS Analysis",
Europ. Mass.
Spectrom. 3, 105-111 (1997). (Original DSI/ChromatoProbe paper)
3. H. Jing and A. Amirav, "Pesticide Analysis with the PFPD and a Novel Direct Sample Introduction Device", Anal. Chem., 69, 1426-1435 (1997).
 4. A. Amirav and H. Jing, "Simultaneous PFPD-MS
Detection for Enhanced Pesticides Analysis Capabilities", J.
Chromatog. A., 814, 133-150 (1998).
4. A. Amirav and H. Jing, "Simultaneous PFPD-MS
Detection for Enhanced Pesticides Analysis Capabilities", J.
Chromatog. A., 814, 133-150 (1998).
5. S. B. Wainhaus, S. Dagan, M. L. Miller and A. Amirav, "Fast Drug Analysis in A Single Hair", J. Am. Soc. Mass. Spectrom., 9, 1311-1320 (1998).
6. A. Amirav and S. Dagan, "Fast GC-MS in Supersonic Molecular Beams", International Laboratory, 17A-17L, March (1996).
7 . A. Amirav, S. Dagan, T. Shahar, N. Tzanani, S.B. Wainhaus, "Fast GC with Supersonic Molecular Beams", Adv. in Mass Spectrom., vol. 14, Chp. 22, 529-562 (1998), E.J. Karjalainen et al., editors, Elsevier, Amsterdam.
8 . A. Amirav and H. Jing, "Pulsed Flame Photometer Detector for Gas Chromatography", Anal. Chem. 67, 3305-3318 (1995).
 9. A. Gordin and A. Amirav., "SnifProbe - A New
Method and Device for Vapor and Gas Sampling", J. Chromatog. A. 903,
155-172 (2000). (Original SnifProbe paper)
9. A. Gordin and A. Amirav., "SnifProbe - A New
Method and Device for Vapor and Gas Sampling", J. Chromatog. A. 903,
155-172 (2000). (Original SnifProbe paper)
10. W. H. Ding, and C. T. Chen., "Analysis of linear alkylbenzenesulfonates in water samples by large-volume injection-port derivatization and gas chromatography-mass spectrometry", J. Chromatog. A. 857, 359-364 (1999).
11. W. H. Ding and C. T. Chen., "Analysis of nonylphenol polyethoxycarboxylates and their related metabolites by on-line derivatization and ion-trap gas chromatography-mass spectrometry" J. Chromatog. A. 862, 113-120 (1999).
12. W. H. Ding and J. C. H. Fann., "Determination of linear alkylbenzenesulfonates in sediments using pressurized liquid extraction and ion-pair derivatization gas chromatography-mass spectrometry" Anal. Chim. Acta. 408, 291-297 (2000).
13. S. J. Lehotay., "Analysis of pesticide residues in mixed fruit and vegetable extracts by direct sample introduction/gas chromatography/tandem mass spectrometry", J. AOAC. INT. 83, 680-697, (2000).
14. W. H. Ding, C. H. Liu and S. P. Yeh, "Analysis of
chlorophenoxy acid herbicides in water by large-volume on-line
derivatization and gas chromatography-mass spectrometry" J. Chromatog.
A. 896, 111-116 (2000).
15. W. H. Ding and C. Y. Wu, "Determination of Estrogenic Nonylphenol and Bisphenol A in River Water by Solid-Phase Extraction and Gas Chromatography -Mass Spectrometry" J. Chinese. Chem. Soc. 47, 1155-1160 (2000).
16. T. Faye, A. Brunot, M. Sablier, J. C. Tabet and T. Fujii, "Sodium ion attachment reactions in an ion trap mass spectrometer" Rapid. Commun. Mass. Spectrom. 14, 1066–1073 (2000).
17. S. Kakimoto, M. Kitagawa and S. Hori, "Rapid and Simple Method for the Analysis of Organophosphorus Pestcides and these Metabolites in the Blood by Applying GC-MS with Chromatoprobe Injector" Japan. J. Food. Chem. 8(3) (2001).
18. A. H. Falkovich and Y. Rudich., "Analysis of semivolatile organic compounds in atmospheric aerosols by direct sample introduction thermal desorption GC/MS" Environ. Sci. Technol. 35, 2326-2333, (2001).
19. S. J. Lehotay, A. R. Lightfield, J. A. Herman-Fetch and D. J. Donoghue "Analysis of Pesticide Residue in Eggs by Direct Sample Introduction/Gas Chromatography/Tandem Mass Spectrometry. J. Agric. Food Chem. 49, 4589-4595 (2001).
20. S. Kakimoto, M. Kitagawa and S. Hori "Rapid and Simple Method for the Analysis of Organophosphorus Pestcides and their Metabolites in the Blood by Applying GC-MS with Chromatoprobe Injector" Japan. J. Food Chem, 8(3), (2001).
22. G. D. McDonald, "Thermal Desorption/GC-MS Analysis of Astrobiologically Relevant Organic Material" Astrobiology 1, 369 (2001).
22. C. Feigel, "Rapid Analysis of Soils for Hazardous Waste by
Direct Sample Introduction"
Varian GC/MS Application note number 56.
http://www.varianinc.com/image/vimage/docs/products/chrom/apps/gcms56.pdf
23. S. Wilkinson, "The Use of a Solids Inlet System to Identify
Essential Oils in Anthers and leaves of Flowering Plants" Varian GC/MS
Application note number 65
http://www.varianinc.com/image/vimage/docs/products/chrom/apps/gcms65.pdf
24. M. Kochman, A. Gordin, P. Goldshlag, S. J. Lehotay and A.
Amirav, "Fast, high-sensitivity, multipesticide analysis of complex
mixtures with supersonic gas chromatography-mass spectrometry" J.
Chromatog. A. 974,
185-212 (2002).
25. W. H. Ding and C. C. Chiang, "Derivatization procedures for the detection of estrogenic chemicals by gas chromatography/mass spectrometry" Rapid Commun. Mass Spectrom. 17, 56-63 (2002).
26. S. H. Tzing, A. Ghule, J. Y. Chang and Y. C. Ling, "Chemical ionization of substituted naphthalenes using tetrahydrofuran as a reagent in gas chromatography with ion trap mass spectrometry" Rapid. Com.Mass. Spectrom.17, 811–815 (2003).
27. S. H. Tzing, A. Ghula, J. Y. Chang and Y. C. Ling, "Selective adduct formation by furan chemical ionization reagent in gas chromatography ion trap mass spectrometry" J. Mass Spectrom. 38, 401-408 (2003).
28. S. H. Tzing, J.Y. Chang, A. Ghule, J.J. Chang, B. Lo and Y. C. Ling, "A simple and rapid method for identifying the source of spilled oil using an electronic nose: confirmation by gas chromatography with mass spectrometry" Rapid. Com.Mass. Spectrom.17, 1873–1880 (2003).
29. K. Patel, R. J. Fussell, D. M. Goodall and B. J. Keely "Analysis of pesticide residues in lettuce by large volume-difficult matrix introduction-gas chromatography-time of flight-mass spectrometry (LV-DMI-GC-TOF-MS)" Analyst 128, 1228-1231 (2003).
30. S. De Koning, G. Lach, M. Linkerhagner, R. Loscher, P. H. Tablack and U. A. T. Brinkman, "Trace-level determination of pesticides in food using difficult matrix introduction-gas chromatography-time-of-flight mass spectrometry" J. Chromatogr. A. 1008, 247-252 (2003).
31. M. Orioli, C. Marinello, R. Cozzi, L.P. Piodi and M. Carini,
"LC-MS/MS
and FT-IR analyses of stones from a patient with Crohn's disease: a
case
report" J. Pharmaceut. Biomed. Anal. 35, 1263-1272 (2004).
32. A. Jurgens and S. Dotterl, "Chemical composition of anther volatiles in
Ranunculaceae: genera-specific profiles in Anemone,
Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species " Am. J. Botany. 91, 1969-1980
(2004).
33. A. Jurgens and S. Dotterl, "Chemical composition of anther volatiles in ranunculaceae: Genera-specific profiles in Anemone, Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species"
34. S. Dotterl and A. Jurgens "Spatial fragrance patterns in flowers of Silene latifolia: Lilac compounds as olfactory nectar guides?" Plant. Systemat Evolut. 255, 99-109 (2005).
35. S. Dotterl, L. M. Wolfe and A. Jurgens "Qualitative and Quantitative analyses of flower scent in Silene Latifolia" Phytochemistry 66, 203-213 (2005).
36. W. C. Lin, H. C. Chen and W. H. Ding, "Determination of pharmaceutical residues in waters by solid-phase extraction and large-volume on-line derivatization with gas chromatography-mass spectrometry" J. Chromatogr. A. 1065, 279-285 (2005).
37. S. Yan, W. Xin, G. Luo, Y. Wang and Y. Cheng, "Chemical Fingerprinting of Gardenia Jasminoides Fruit Using Direct Sample Introduction and Gas Chromatography Mass Spectrometry Detection" J. AOAC Int. 89, 40-45 (2006).
38. A. Jurgens, H. Feldhaar, H. Feldmeyer and B. Fiala, "Chemical composition of leaf volatiles in Macaranga species (Euphorbiaceae) and their potential role as olfactory cues in host-localization of foundress queens of specific ant partners" Biochem. Systemat. Ecol. 34, 97-113 (2006).
39. C. Y. Cheng, W. R. Li, J. W. Chang, H. C. Wu and W. H. Ding "Synthesis and determination of dicarboxylic degradation products of nonylphenol polyethoxylates by gas chromatography–mass spectrometry" J. Chromatogr. A. 1127, 246-253 (2006).
40. K. Mastovska and S. J. Lehotay "Rapid sample preparation method for LC-MS/MS or GC-MS analysis of acrylamide in various food matrices: J. Agricul. Food Chem. 54, 7001-7008 (2006).
41. M. Poliak, M. Kochman, A. Gordin and A. Amirav "A Comparison of SnifProbe and SPME for Aroma Sampling" Chromatographia, 67, 487-493 (2006).
42. A. Jurgens, S. Dottrel and U. Meve "The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae)" New Phytologist 172, 452-468 (2006).
43. A. Jürgens, H. Feldhaar, B. Feldmeyer and B. Fiala, Chemical composition of leaf volatiles in Macaranga species (Euphorbiaceae) and their potential role as olfactory cues in host-localization of foundress queens of specific ant partners" Biochem. System. Ecology, 34, 97-113 (2006).
44. S. H. Tzing, A. Ghule, J. Y. Liu and Y. C. Ling, "On-line derivatization gas chromatography with furan chemical ionization tandem mass spectrometry for screening of amphetamines in urine" J. Chromatogr. A. 1137, 76-83 (2006).
45. K. Mastovska and S. J. Lehotay "Rapid sample preparation method for LC-MS/MS or GC-MS analysis of acrylamide in various food matrices" J. Agricul Food Chem. 54, 7001-7008 (2006).
46. C.Y. Cheng, C.Y. Wu, C. H. Wang and W. H. Ding "Determination and distribution characteristics of degradation products of nonylphenol polyethoxylates in the rivers of Taiwan" Chemosphere, 65, 2275-2281 (2006).
47. C. Y. Cheng, L. L. Liu and W. H. Ding, "Occurrence and seasonal variation of alkylphenols in marine organisms from the coast of Taiwan" Chemosphere, 65, 2152-2159 (2006).
48. S. Dötterl and I. Schäffler, "Flower Scent of Floral Oil-Producing Lysimachia punctata as Attractant for the Oil-Bee Macropis fulvipes" J. Chem. Ecology, 33, 441-445 (2007).
49. C.Y. Cheng and W. H. Ding, "Determination of acidic degradation products of nonylphenol polyethoxylates by large-volume injection-port derivatization gas chromatography/mass spectrometry" Rapid. Commun. Mass Spectrom. 21, 1687-1690 (2007).
50. C. L. Hsu, C. Y. Cheng, C.T. Lee and W. H. Ding "Derivatization procedures and determination of levoglucosan and related monosaccharide anhydrided in atmospheric aerosols by gas chromatography-mass spectrometry" Talanta 72, 199-205 (2007).
51. U. Fussel, S. Dotterl and A Jurgens, Inter-and Intraspecific Variation in Floral Scent in the Genus Salix and it Implications for Pollination" J. Chem. Ecol. 33, 749-765 (2007).
52. U. S. Jhumur, S. Dötterl and A. Jürgens, "Floral Odors of Silene otites: Their Variability and Attractiveness to Mosquitoes" J. Chem. Ecology, 34, 14025 (2007).
54. L. G. Benavides, S. Dötterl, A. Jürgens, A. Escudero and J. M. Iriondo, "Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene – Hadena interaction" Oicos 116, 1461-1472 (2007).
55. T. J. Benson, W. E. Holmes, M. G. White, W. T. French, E. G. Alley and R. Hernandez, "Development of a heterogeneous catalytic cracking reactor utilizing online mass spectrometry analysis" J. Chromatogr. A. 1172, 204-208 (2007).
56. W. Cummins, P. Duggan and P. McLoughlin "Thermal Desorption Characterization of Molecularly Imprinted Polymers. Part I: a Novel Study Using Direct-Probe GC-MS Analysis" Anal. Bioanal. Chem. 391, 1237-1244 (2008).
57. N. Holland, P. Duggan, E. Owens, W. Cummins, J. Frisby, H. Hughes and P. McLoughlin, "Thermal desorption characterisation of molecularly imprinted polymers. Part II: Use of direct probe GC-MS analysis to study crosslinking effects" Anal. Bioanal. Chem. 391, 1245-1253 (2008).
58. E. Hoh and K. Mastovska, Large volume injection techniques in capillary gas chromatography" J. Chromatogr. A. 1186, 2-15 (2008).
59. A. M. El-Sayed, J. A. Byers, L. M. Manning, A. Jürgens, V. J. Mitchell, and D. M. Suckling, "Floral Scent of Canada Thistle and Its Potential as a Generic Insect Attractant" J. Economic Entomology, 101, 720-727 (2008).
60. H. Truong, S. Lomnicki and B. Dellinger, "Mechanisms of molecular product and persistent radical formation from the pyrolysis of hydroquinone", Chemosphere 71, 107-113 (2008).
61. A. Amirav, A. Gordin, M. Poliak and A. B. Fialkov "Gas chromatography-mass spectrometry with supersonic molecular beams" J. Mass Spectrom. 43, 141-163 (2008).
62. S. Lomnicki, H. Truong and B. Dellinger, "Mechanisms of product formation from the pyrolytic thermal degradation of catechol" Chemosphere 73, 629–633 (2008).
63. A. Shuttleworth and S. D. Johnson, A key role for floral scent in a wasp-pollination system in Eucomis (Hyacinthaceae)" Annals of Botany 103, 715-725 (2008).
64. E. Hoh, S. J. Lehotay, K. Mastovska and J. K. Huwe, "Evaluation of automated direct sample introduction with comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for the screening analysis of dioxins in fish oil" J. Chromatogr. A, 1201, 69–77 (2008).
65. U. S. Jhumur, S. Dötterl and A. Jürgens, "Floral Odors of Silene otites: Their Variability and Attractiveness to Mosquitoes" J. Chem. Ecology, 34, 14-25, (2008).
66. M. Diaby, S. Kinani, C. Genty, S. Bouchonnet, M. Sablier, A. Le Negrate, and M. El Fassi "Analysis of the Volatile Organic Matter of Engine Piston Deposits by Direct Sample Introduction Thermal Desorption Gas Chromatography/Mass Spectrometry" Anal. Chem. 81, 9764-9770 (2009).
67. E. Hoh, S.J. Lehotay, K. Mastovska, H. L Ngo, W. Vetter, K. C. Pangallo and C. M. Reddy "Capabilities of Direct Sample Introduction-Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry to Analyze Organic Chemicals of Interest in Fish Oils" Environ. Sci Tech. 43, 3240-3247 (2009).
68. A. Livingston, E. Robinson, R. A. Armitage, "Characterizing the binders in rock paintings by THM-GC–MS: La Casa de Las Golondrinas, Guatemala, a cautionary tale for radiocarbon dating" Int. J. Mass Spectrom. 284, 142–151 (2009).
69. M. Proffit and S.D. Johnson "Specificity of the signal emitted by figs to attract their pollinating wasps: Comparison of volatile organic compounds emitted by receptive syconia of Ficus sur and F. sycomorus in Southern Africa" S. African J. Botany, 75, 771-777 (2009)
70. M. Feulner, F. Schuhwerk and S. Dotterl "Floral scent analysis in Hieracium subgenus Pilosella and its taxonomical implications" Flora 204, 495–505 (2009).
71. A. Jurgens, S. Dotterl, S. Liede-Schumann and U. Meve "Chemical diversity of floral volatiles in Asclepiadoideae-Asclepiadeae (Apocynaceae)" Biochem. Systematics & Ecology 36, 842–852 (2009).
72. D. A. Perry, J. S. Cordova, L. G. Smith, H. J. Son, and E. M. Schiefer, "Study of Adsorption of Aminobenzoic Acid Isomers on Silver Nanostructures by Surface-Enhanced Infrared Spectroscopy" J. Phys. Chem. C 113, 18304–18311 (2009).
73. S. Proches and S. D. Johnson "Beetle pollination of the fruit-scented cones of the South African cycad Stangeria eriopus" Am. J. Botany 96, 1722-1730 (2009).
74. D. Perry, J, Boucher, K, Posey, S, Cordova, L, Smith, H, J, Son, R, Pandey and A. S. Biris, "Surface-enhanced spectroscopic investigation of the adsorption properties of hydroxybenzoic acid isomers onto metallic surfaces" Spectrochimica Acta A. 74, 104-112 (2009).
75. S. D. Johnson, M. E. Griffiths, C. I. Peter and M. J. Lawes, "Pollinators, "mustard oil" volatiles, and fruit production in flowers of the dioecious tree Drypetes natalensis (Putranjivaceae)" Am. J. Botany 96, 2080-2086 (2009).
76. H. W. Chung and W. H. Ding, "Determination of organophosphate flame retardants in sediments by microwave-assisted extraction and gas chromatography–mass spectrometry with electron impact and chemical ionization" Anal Bioanal Chem 395, 2325–2334 (2009).
77. D. Perrya, J. Bouchera, K. Poseya, S. Cordovaa, L. Smitha and H. J. Sona, "Surface-enhanced spectroscopic investigation of the adsorption properties of hydroxybenzoic acid isomers onto metallic surfaces" Spectrochimica Acta Part A 74, 104–112 (2009).
78. S. P. Huang, P. S. Chen, S. D. Huang "Dynamic headspace time-extended helix liquid-phase microextraction" J. Chromatogr. A, 1216, 4347–4353 (2009).
79. C. L. Hsu and W. H. Ding "Determination of low-molecular-weight dicarboxylic acids in atmospheric aerosols by injection-port derivatization and gas chromatography–mass spectrometry" Talanta, 80, 1025-1028 (2009).
80. M. Proffit and S. D. Johnson, "Specificity of the signal emitted by figs to attract their pollinating wasps: Comparison of volatile organic compounds emitted by receptive syconia of Ficus sur and F. sycomorus in Southern Africa" S. African J. Botany, 75, 771-777 (2009).
81. A. Jurgens, A. M. El-Sayed and D. M. Suckling, "Do
carnivorous plants use volatiles for attracting prey insects?"
Func. ECOLOGY, 23,
875-887 (2009)
82. A. Shuttleworth and S. D. Johnson, "The
importance of scent and nectar filters in a specialized wasp-pollination system"
Func. Ecology 23,
931-940 (2009).
83. S.
Dotterl, A.
Jurgens, L.
Wolfe and A.
Biere, "Disease
Status and Population Origin Effects on Floral Scent: Potential Consequences for
Oviposition and Fruit Predation in A Complex Interaction Between A Plant,
Fungus, and Noctuid Moth" J. Chem.
Ecology, 35, 307-319 (2009). 84. S. H. Tzing,
W. H. Ding "Determination of melamine and cyanuric acid in powdered milk using
injection-port derivatization and gas chromatography–tandem mass spectrometry
with furan chemical ionization" J. Chromatogr. A, 1217, 6267–6273 (2010)
85. A. Heiduk, I. Brake, T. Tolasch, J. Frank, A. Jürgens, U. Meve and S.
Dötterl "Scent chemistry and pollinator attraction in the deceptive trap flowers
of Ceropegia dolichophylla" S. African J. Botany 76, 762–769 (2010).
86. H. Burger, M. Ayasse, C. M. Haberlein, S. Schulz and S. Dotterl "Echium
and Pontechium specific floral cues for host–plant recognition by the
oligolectic bee Hoplitis adunca" S. African J. Botany 76, 788–795 (2010).
87. T. N. Suinyuy, J. S. Donaldson and S. D. Johnson "Scent chemistry and
patterns of thermogenesis in male and female cones of the African cycad
Encephalartos natalensis (Zamiaceae)" S. African J. Botany 76, 717–725 (2010).
88. N. Holland, J. Frisby, E. Owens, H. Hughes, P. Duggan and P. McLoughlin
"The influence of polymer morphology on the performance of molecularly imprinted
polymers" Polymer 51, 1578–1584 (2010).
89. A. Shuttleworth and S. D. Johnson "Floral scents of chafer-pollinated
asclepiads and a potential hybrid" S. African J. Botany 76, 770–778 (2010).
90. A. Jürgens, S. Dötterl, S. Liede-Schumann and U. Meve "Floral scent
composition in early diverging taxa of Asclepiadoideae, and Secamonoideae (Apocynaceae)"
S. African J. Botany 76, 749–761 (2010).
91. S. D. Johnsona and N. Hobbhahna, "Generalized pollination, floral scent
chemistry, and a possible case of hybridization in the African orchid Disa
fragrans" S. African J. Botany 76, 739–748 (2010).
92. S. L. Steenhuisen, R. A. Raguso, A. Jürgens and S. D. Johnson "Variation
in scent emission among floral parts and inflorescence developmental stages in
beetle-pollinated Protea species (Proteaceae)" S. African J. Botany 76, 779–787
(2010).
93. T. Van der Niet, A. Jürgens and S. D. Johnson "Pollinators, floral
morphology and scent chemistry in the southern African orchid genus Schizochilus"
S. African J. Botany 76, 726–738 (2010).
94. M. J. Kotze, A. Jürgens, S. D. Johnson and J. H. Hoffmann, "Volatiles
associated with different flower stages and leaves of Acacia cyclops and their
potential role as host attractants for Dasineura dielsi (Diptera: Cecidomyiidae)"
S. African J. Botany 76, 701–709 (2010).
95. D. A. Perry, H. J. Son, J. S. Cordova, L. G. Smith and A. S. Biris,
"Adsorption analysis of nitrophenol isomers on silver nanostructures by
surface-enhanced spectroscopy" J. Colloid Interface Sci. 342, 311–319 (2010).
96. S. D. Johnson and A. Jürgens "Convergent evolution of carrion and faecal
scent mimicry in fly-pollinated angiosperm flowers and a stinkhorn fungus" S.
African J. Botany 76, 796–807 (2010). 97.
M. C. Martinell,
S. Dötterl,
C. Blanché,
A. Rovira,
S. Massó and
M. Bosch, "Nocturnal
pollination of the endemic Silene sennenii (Caryophyllaceae): an
endangered mutualism?"
Plant Ecology,
211,
203-218, (2010).
98. A.
Kehl, S.
Dotterl, G.
Aas and G.
Rambold, "Is flower scent influencing
host plant selection of leaf-galling sawflies (Hymenoptera, Tenthredinidae) on
willows?" Chemoecology, 20, 215-221 (2010). 99. K.
Mastovska, K. J.
Dorweiler, S. J.
Lehotay, J. S.
Wegscheid, and K. A.
Szpylka, "Pesticide Multiresidue
Analysis in Cereal Grains Using Modified QuEChERS Method Combined with Automated
Direct Sample Introduction GC-TOFMS and UPLC-MS/MS Techniques" J. Agri. Food
Chem., 58, 5959-5972 (2010).
100. B. Anderson, R. Alexandersson and S. D. Johnson "Evolution and
Coexistence of Pollination Ecotypes in the African Gladiolus (Iridaceae)
EVOLUTION, 64, 960-972 (2010). 101.
D. Piechowski, S. Dötterl and G. Gottsberger, "Pollination biology and floral
scent chemistry of the Neotropical chiropterophilous Parkia pendula"
Plant Biology,
12, 172–182, (2010).
102.
J. Jersáková,
S. Castro,
N. Sonk,
K. Milchreit,
I. Schödelbauerová,
T. Tolasch and
S. Dötterl, "Absence
of pollinator-mediated premating barriers in mixed-ploidy populations of
Gymnadenia conopsea s.l. (Orchidaceae)"
Evolutionary Ecology,
24,
1199-1218,
(2010).
103.
A. Shuttleworth and
S. D. Johnson, "The missing stink:
sulphur compounds can mediate a shift between fly and wasp pollination systems"
Proc. Royal Soc. B. (2010).
104. D. A. Perrya, J. S. Cordovaa, L. G. Smith, H. J. Sona and A. S. Biris
"Characterization of aminophenol isomer adsorption on silver nanostructures"
Vibrational Spectroscopy 55, 77–84 (2011).
105. F. Balao, J. Herrera, S
Talavera and S. Dötterl, "Spatial
and temporal patterns of floral scent emission in Dianthus inoxianus and
electroantennographic responses of its hawkmoth pollinator" Phytochemistry,
72, 601-609 (2011).
107.
D. A. Perry, H. J. Son, J. S. Cordova, L.
G. Smith and A. S. Biris, "Adsorption
analysis of nitrophenol isomers on silver nanostructures by surface-enhanced
spectroscopy" J. Colloid and Interface Sci., 342, 311-319 (2010)
108. C. Y. Cheng,
Y. C. Wang, H. C. Chen and W. H. Ding "Simplified Derivatization Method for
Triclosan Determination in Personal Care Products by Gas Chromatography-Mass
Spectrometry, J. Chinese Chem. Soc., 58, 49-52 (2011). 109. C. Y. Cheng,
Y. C. Wang, H. C. Chen and W. H. Ding
"Determination of Triclosan in Aqueous Samples Using Solid-phase Extraction
Followed by On-line Derivatization Gas Chromatography–Mass Spectrometry"
Anal. Sci. 27, 197 (2011). 110 R. R. Junker, S. Bretscher, S. Dötterl and N.
Blüthgen, "Phytochemical cues affect hunting-site choices of a nursery web
spider (Pisaura mirabilis) but not a crab spider (Misumena vatia)" J.
Arachnology, 39,113-117 (2011). 111. Y. C. Ho and W. H. Ding "Solid-phase Extraction Coupled Simple
On-line Derivatization Gas Chromatography Tandem Mass Spectrometry for the
Determination of Benzophenone-type UV Filters in Aqueous Samples" J. Chin.
Chem. Soc., 58, 6, 2011. 112. A. O. Olaniran, Y. R. Maharaj and B. Pillay "Effects of fermentation
temperature on the composition of beer volatile compounds, organoleptic
quality and spent yeast density" E. J. Biotech. 14, (2011). 113. T. van der Niet, D. M. Hansen and S. J. Johnson, "Carrion
mimicry in a South African orchid-flowers attract a narrow subset of the fly
assemblage on animal carcasses" Annals of Botany 107, 981-992 (2011). 114. E. G. Bowes, G. M. Lee, C. M. Vogels, A. Decken and S. A. Westcott,
"Palladium
salicylaldimine complexes derived from 2,3-dihydroxybenzaldehyde" Inorganica
Chimica Acta, In Press (2011). 115. M. Feulner, F. Schuhwerk and S. Dötterl "Taxonomical
value of inflorescence scent in Hieracium s. str."Biochem. Systematics
and Ecology, In Press (2011). 116. R. R. Junker, C. Loewel, R. Gross, S. Dötterl, A. Keller and N.
Blüthgen, "Composition of epiphytic bacterial communities differs on petals
and leaves" Plant Biology (2011).
106. A. Shuttleworth, S.D. Johnson. "Floral
scents of chafer-pollinated asclepiads and a potential hybrid" South
African J. Botany, 76, 770-778 (2010).
For further ChromatoProbe and/or SnifProbe information or advice, please contact me through my E-mail: amirav@tau.ac.il
Further information on the Aviv Analytical ChromatoProbe can be obtained by clicking on its link.
Further information on the Aviv Analytical SnifProbe can be obtained by clicking on its link.