The Method of Standard Additions
In many cases the intensity of the signal of the analyte is affected by the composition of the matrix, by the temperature and other factors.
One of the methods to overcome these problems is the method of standard additions. Two conditions have to be fulfilled for successful application of the method:
(a) the calibration graph must be linear,
(b) the calibration curve of the analyte passes through the origin.
The signal intensity of the sample solution is measured and then portions of a solution of the element at a known concentration are added and the signal intensity is measured after each addition. The optimal size of each addition is that which gives a signal 1.5 to 3 times that of the sample.
For each addition:
![]()
where Cx and (Cx + Cs) are the concentration of the analyte without and with the standard addition, respectively,
signalx and signalx+s are the signal intensities of the solutions containing Cx and (Cx + Cs).
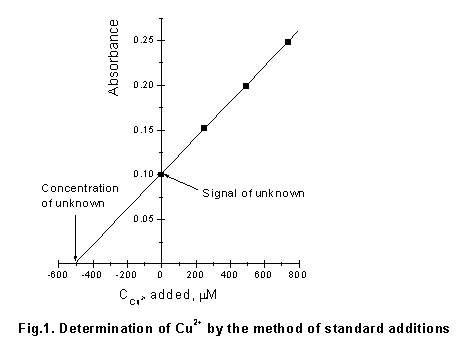
A plot of the signal intensities of the solutions vs. the added concentrations yields a straight line. The concentration of the analyte is determined from the point at which the extrapolated line crosses the concentration axis at zero signal.
An example is given in Table 1 and Fig.1.
Table
1. Data for the determination of Cu2+ by the standard-addition method.
Sample volume -
20.00 ml; concentration of the Cu2+ solution used for standard
additions - 0.5 mM.
|
Solution No. |
Volume of 0.5 mM Cu2+ added, |
Concentration of the standard addition in the
cell, |
Signal (absorbance) |
|
1 2 3 4 |
0 100 200 300 |
0 249 495 739 |
0.100 0.152 0.199 0.248 |
Two modes of preparing the solutions are used, depending on the type of cell used in a specific analysis:
(a) The analyte and mixtures of analyte with the respective standard additions are prepared in separate volumetric flasks.
(b) A known amount of analyte is introduced into a cell (with or without additions of known volumes of other constituents). The volume of the standard additions is small so as not to change the matrix composition of the sample. The analytical signal is recorded. Standard additions are made in succession. The changes in the concentration of the analyte as a result of the additions must be calculated.
The main advantage of the method is that the matrix remains unchanged. The method is suitable for cases in which the matrix is complex or is difficult to reproduce.
The method of sample bracketing
If the calibration graph is curved, the standards are chosen so that they are very close to the concentration of the unknown. After the initial run of the unknown, two standards are chosen - one with a concentration lower than the unknown and one higher. The segment between the two standards can be considered linear and an accurate determination can be realized.
The method is suitable for the case of non-linear calibration curves and when reasonable matching of the matrix can be achieved.