EDTA
Ethylenediaminetetraacetic acid (also called ethylenedinitrilotetraacetic acid), which is commonly shortened to EDTA, has the structure:
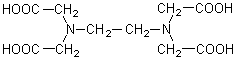
The dissociation constants for the four carboxylic acid groups are: K1 = 1.02·10-2, K2 = 2.14·10-3, K3 = 6.92·10-7 and K4 = 5.50·10-11.
It is of interest that the first two constants are of the same order of magnitude, which suggests that the two protons involved in dissociate from opposite ends of the rather long molecule. As a consequence of their physical reparation, the negative charge created by the first dissociation does not greatly inhibit the removal of the second proton. The same cannot be said for the dissociation of the other two protons, however, which are much closer to the negatively charged carboxylate ions created by the initial dissociations.
Note that the molecule has six potential sites for bonding a metal ion: the four carboxyl groups and the two amino groups, each of the latter with an unshared pair of electrons. Thus, EDTA is a hexadentate ligand.
The various EDTA species are often abbreviated H4Y, H3Y-, H2Y2-, HY3- and Y4-.
The free acid, H4Y, and the dihydrate of the sodium salt, Na2H2Y·2H2O, are available in reagent quality.
Skoog, West and Holler, p. 259.