Flow Cytometry
Flow cytometry is a method for sorting and counting cells according to various characteristics. Either light scattered by the cells or fluorescence emissions from the cells can be detected and measured.
The cells are suspended in a liquid and passed down a narrow channel through a beam of light, with signals generated when they pass individually. Separate measurements of each cell are detected and computed so that the results diplayed represent cumulative individual cell cytometric characteristics.
The scattered light detected in the same direction as the incident light is related to cell size, and known as forward scatter. The scattered light collected at a 90 degree angle is related to cell complexity and is known as side scatter. An analogy would be football fans passing down a narrow corridor into a stadium. These fans would have various sizes (large, small) as well as complexity (type of clothing).
The cells can be stained with different fluorochromes. Fluorochromes can bind to specific cell components such as proteins (fluorescein isothiocyanate, or FITC). Fluorochromes can react depending on cellular physiological characteristics (pH, membrane potential, etc.). Fluorochromes can react to cell enzymatic activity (esterases, peroxidases). Fluorochromes can be conjugated to antibodies or nucleotide probes to react with specific cell antigens or cellular RNA or DNA sequences. In the football fan analogy here, the fans would "mark" with clothing having team colours. Those fans having just a cap would mark less strongly than those more fanatic with a shirt and/or pants with the same colour. More than one team colour could be detected, either on separate fans or on the same fans.
The data collected are usually displayed as either a monoparametric or a biparametric histogram.
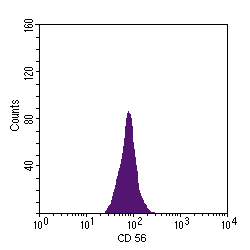
The monoparametric histogram displays a measured parameter on the x axis and the relative cell number on the y axis, as shown below with marking of cells for CD 56, an NK cell marker:
The biparametric histogram displays cells distributed as a function of their signal intensity with respect to each measured parameter. The results appear in four quadrants, with increasing intensity (on a log scale) along each axis.
Cell data appearing in the upper left quadrant represent the parameter measured on the y axis.
Cell data in the lower right quadrant represent the parameter measured on the x axis.
Cell data in the upper right quadrant represent positivity for both measured parameters.
Cell data in the lower left quadrant represent double negativity for both parameters.
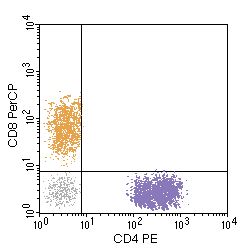
In the above example, separate peripheral blood T cell populations are detected, those helper cells (CD4 positive) coloured purple and those suppressor cells (CD8 positive) coloured gold with a population shaded in grey not marking (B cells, NK cells). No cells in the peripheral blood mark for both. The number of dots gives an indication of the size of each population marking in the sample tested.

In the above example, a large population of cells marks with CD34 (green), typical for myeloblasts, while another smaller population of normal mature myeloid cells also marks for CD64 (red).
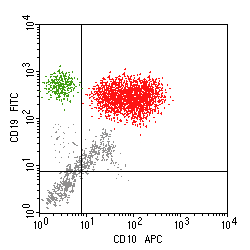
In the above example, there is a large population of cells marking strongly with both CD10 and CD19 (red), indicative of a B cell follicular lymphoma, and a smaller population of normal B cells (green).
