Immunohistochemistry
The use of immunohistochemical methods greatly aids in diagnosis of many pathologic conditions in which tissue is obtained at surgery, from biopsy, via cytologic sampling, or at autopsy. Immunohistochemical techniques make use of an antigen-antibody reaction coupled with a reaction that produces a chromogen (colored product) or attachment of a fluorescein dye to identify specific components in tissues. Most methods are "indirect" because two antibodies are utilized:
Primary antibody: an antibody is raised to a specific tissue component that acts as an antigen, such as antibody to cytokeratin or vimentin or an infectious agent within the tissue such as viral hepatitis B or Histoplasma. This primary antibody attaches via its Fab portion (which is the part with antigenic specificity) to the tissue antigen. The primary antibody is typically from a non-human mammalian source such as mouse or goat or rabbit.
Secondary antibody: an antibody is raised to the Fc portion (the constant part) of the primary antibody. It is raised via another mammalian species which will consider the primary antibody as a foreign antigen and make antibody to it. It is this secondary antibody that has a marker attached to it that will provide visibility.
The use of this indirect method is efficient, because a variety of primary antibodies can be manufactured, but only a single secondary antibody with its marker needs to be produced, since attaching the marker can yield variable results with different antibodies. The primary antibodies are typically "monoclonal" antibodies which are all the same, with the same specificity for a particular antigen.
These monoclonal antibodies can be made by immunizing a mouse with the antigen to be detected, then taking the B-lymphocytes from the mouse spleen and fusing them with cells from a myeloma--a form of malignant neoplasm composed of plasma cells that make antibody. This fusion imparts the antibody specificity of the mouse B-lymphocytes along with the "immortality" of the cultured myeloma cells that continue to proliferate and make the same antibody over and over again.
The markers attached to the secondary antibody can be chromogens that can be viewed by light microscoopy or they can be fluorescein dyes seen via fluorescent light microscopy. In the case of the former, the chromogen is produced via reaction with peroxidase. The peroxidase utilized is horseradish peroxidase.
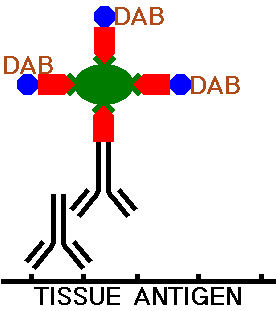
The peroxidase utilized must be attached to the secondary antibody, and it is the localization of this peroxidase in the tissues that provides the ability to identify specific components. The peroxidase must act upon a compound to produce the chromogen. Most ofen, the compound utilized is diaminobenzidine tetrahydrochloride (DAB). A visible insoluble dark brown precipitate is formed by DAB in the presence of peroxidase.
Avidin-Biotin Congugate (ABC) Method
In this method, a primary antibody specific for a particular tissue antigen is utilized. The antibody is "titered" (diluted) to an optimum point where visible staining can be achieved while using as little expensive antibody as possible. Formalin-fixed, paraffin-embedded blocks of tissue are sectioned on a microtome and a thin section placed on a glass slide. Tissue fixation with 10% neutral buffered formalin tends to preserve antigens in the tissue quite well. The section is deparaffinized, and the tissue is pre-treated with hydrogen peroxide to remove any endogenous peroxidase enzyme activity inherent in the tissue that might produce non-specific staining.
The tissue section is then incubated with the primary antibody, which will attach to any specific tissue antigen present as shown below:

The section is then washed to remove any unbound primary antibody. Then the secondary antibody, which is typically another mammalian species directed against the species of the primary antibody, is applied to the tissue section. This secondary antibody is "biotinylated" or complexed with biotin, a low molecular weight vitamin compound. The tissue is incubated with this secondary antibody, which will attach to any specific primary antibody that is present, as shown below:
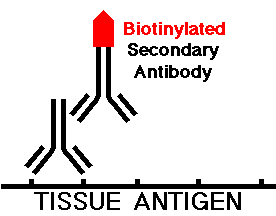
The tissue section is washed to remove any excess secondary antibody. An avidin-biotin complex is then added. Avidin, a protein derived from egg-white, has a very high affinity for biotin and can form complexes with multiple copies of biotin. The other component of the complex is biotin with the horseradish peroxidase enzyme attached, which can also complex with the avidin.
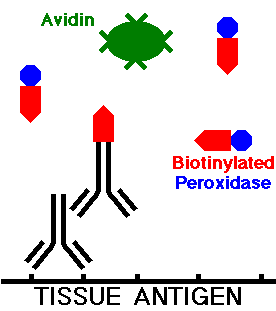
During incubation, the avidin then complexes with the biotinylated secondary antibody, localizing it to a specific site in the tissue, and biotin with peroxidase also attaches to the avidin. Thus, the avidin-biotin-conjugate (ABC) complex is formed, as illustrated below:
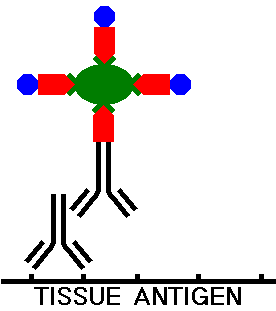
The tissue section is next washed to remove any excess ABC complexes. The the tissue on the slide is then incubated with a solution containing the reagent diaminobenzidine (DAB) which will react with the peroxidase and form the chromogen, making the areas of tissue with antibody binding visible under light microscopy.
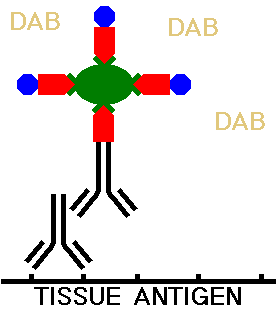
The DAB reagent reacts with the peroxidase to produce an insoluble dark brown precipitate that is localized to the area of the tissue with the antigen to be identified. A counterstain such as hematoxylin can be applied to make the histologic features of the section, such as nuclei and cell cytoplasm, more apparent, and provide a contrast with the dark brown DAB chromogen. This allows the location of the staining to be determined.
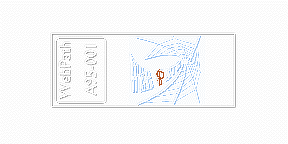
In the diagrammatic representation below, the end result of immunohistochemical staining with the ABC method is shown, with an "anti-letter P" antibody having been applied to the section of "WebPath" on the slide.
|
 Return to the Anatomy-Histology main menu.
Return to the Anatomy-Histology main menu.
 Return to the Anatomy-Histology main menu.
Return to the Anatomy-Histology main menu.