Autosomal Recessive Inheritance - Examples
Pedigree
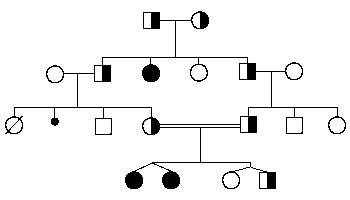
Note that carriers do not manifest the disease. The trait is on an autosome and can be passed to offspring who are males or females. Note that consanguinity can increase the risk for appearance of an autosomal recessive trait in a family.
Cystic Fibrosis
An example of a single gene that can have multiple different mutations (alleles) resulting in a similar phenotype is cystic fibrosis. Mutations occur in the CFTR (cystic fibrosis transmembrane conductance regulator) gene. The gene codes for a chloride channel protein that controls chloride ion movement across epithelial cells. CFTR mutations lead to production of abnormally viscid mucoid secretions that lead to organ abnormalities, particularly in the lungs. Since multiple organs are involved, the CFTR gene exhibits pleiotropy. CFTR is also an example of how many different mutations can appear clinically similar and confound screening strategies. There are over 700 known CFTR mutations. The deltaF508 mutation accounts for two-thirds of all mutations. About 12 mutations account for 90%. Thus, you can't just apply a single genetic screening test. Also, even though this is one of the most common inherited abnormalities (1 in 25 Caucasians carries this gene), general population screening is more likely to yield false poaitives. The standard strategy is to screen only when a family history is present or a new case is diagnosed in a family.
Sickle Cell Anemia
An example of a common autosomal recessive condition is sickle cell anemia. Normal adult hemoglobin is comprised of four globin chains that bind iron. Two of the chains are alpha and two are beta. A point mutation in the beta globin chain gene leads to an abnormal globin that causes red blood cells to change shape (sickle) under low oxygen concentrations. The mutation causes a substitution of valine for glutamic acid at position 6 of the beta globin chain, leading to abnormal conformation of this protein.
If one maps the population distribution of Hgb S, it overlaps that of malaria, specifically the most virulent form. Persons heterozygous for Hgb S are partially protected from infection of red blood cells by malarial parasites. Homozygotes die at a young age (without medical care). Thus, there is a biologic selection for Hgb S because of the selective advantage for heterozygotes. In history, such a mutation arose and spread in two populations: central Africa and eastern Arabia.


