

Thoracic anatomical changes during heart contraction reflect on the conductivity distribution therefore making bio-impedance techniques ideal candidates for cardiac Stroke Volume (SV) monitoring. Due to the physics of electrical current propagation in a volume conductor, 3D modeling is necessary, though holds computing and modeling complications. The surface potential can be calculated from the electrical impedance distribution using Poisson's equation:![]() in what is known as the forward problem. In some applications, the inverse relationship is solved using the forward solver. Using a hybrid phantom, 3D realistic simulation infrastructure for solving the forward problem was developed and the solution attributes have been researched. The research objective is to simulate the physical problem in a more realistic way then past studies and then examine the mutual influence of lung conductivity and heart volume on the surface potentials, where its results could benefit future inverse problem solving and SV non-invasive monitoring.
in what is known as the forward problem. In some applications, the inverse relationship is solved using the forward solver. Using a hybrid phantom, 3D realistic simulation infrastructure for solving the forward problem was developed and the solution attributes have been researched. The research objective is to simulate the physical problem in a more realistic way then past studies and then examine the mutual influence of lung conductivity and heart volume on the surface potentials, where its results could benefit future inverse problem solving and SV non-invasive monitoring.
Cardiac cycle was simulated for normal patients and patients with cardiogenic pulmonary edema, taking into consideration the pulmonary blood perfusion during heart contraction. It was found that the forward problem is most sensitive to SV if injecting current from the right breast into the left scapula. The simulations show that both heart volume and lung conductivity effect the developing voltage, meaning that in SV estimation, lung conductivity and the heart volume should be jointly estimated the and not only the latter. The lung conductivity is a nuisance parameter that has to be taken into account. The most sensitive results were achieved by an injection from the right front part of the torso, diagonally into the left scapula. The heart and lungs are the major influencers on thoracic impedance. The heart contains highly conductive blood and the lungs are filled with highly resistive air.
Pathological dilation of the heart would lower the whole thoracic impedance and pulmonary edema would increase it. Since congestive heart failure patients frequently suffer from both lower stroke volume (large end diastolic volume) and fluid retention in the lungs, the joint estimation of both parameters is of major significance.
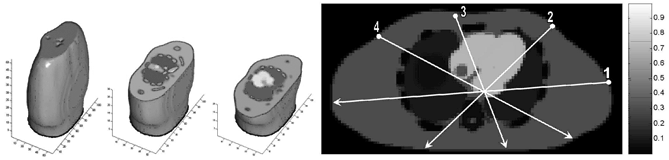
Left figure: Current injections visualization on a 2d transverse impedance map.
Right figure: 3D impedance maps from the NCAT phantom
