Fetal development into a healthy baby is largely dependent on adequate feto-placental perfusion, which provides the required oxygen and nutrients. Placenta insufficiency due to high placental vascular resistance may result in a small for gestational age baby, which is prone to higher risks of morbidity and mortality. The feto-placental vasculature includes the relatively large chorionic vessels, the intra-placental vessels and the capillaries within the cotyledons, where gas and nutrition exchange occurs. The existing mathematical models for simulations of blood flow in the placental vasculature are mostly simplified networks of resistors and capacitors that represent the vesselís resistance and compliance, respectively. In order to extend the scope of prenatal care, we investigated the structure of placental vasculature from cast models and have started to develop physical models of placental fetal circulation.
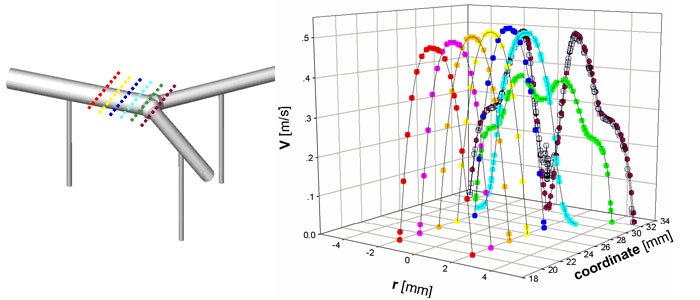
The computational analysis of feto-placental blood flow was performed on simple three-dimensional units of dichotomous (i.e., disperse) and monopodial (i.e., magistral) branching networks of the chorionic arteries with intra-placental (IP) arteries that branch off at angles of 900 with the chorionic plane. The analysis of blood flow pattern in this complex geometry was conducted by implementing the finite volume numerical software of FLUENT for steady fetal blood flow and no-slip conditions on the walls. We assumed diameters of 4 mm and 1 mm for the main chorionic artery and the intra-placental arteries, respectively. The inlet velocity was set to 0.32 m/s based on clinical data.
The resulted predictions in the chorionic plane showed a parabolic distribution with peak velocities up to 0.5 m/s. The relatively large ratio between diameters of the chorionic and IP vessels yields reasonable blood flow at peripheral ends of the chorionic plate and ensures a uniform blood perfusion of the placenta. The results revealed that local occlusions in the IP vessel has little effect on perfusion of other vessels. The architecture of placental blood vessels differs from that of the systematic circulation by the relatively large ratio (e.g., up to an order of magnitude) between the diameters of chorionic vessels and the corresponding intra-placental vessels that branch off them. As a result, the main intra-placental vessels, which perfuse the placenta cotyledons, maintain a relatively high blood flow even at the peripheral ends of the chorionic plate. This architecture ensures a uniform blood perfusion of the placenta for optimal performance of its gas and nutrition exchange functions.