|
|
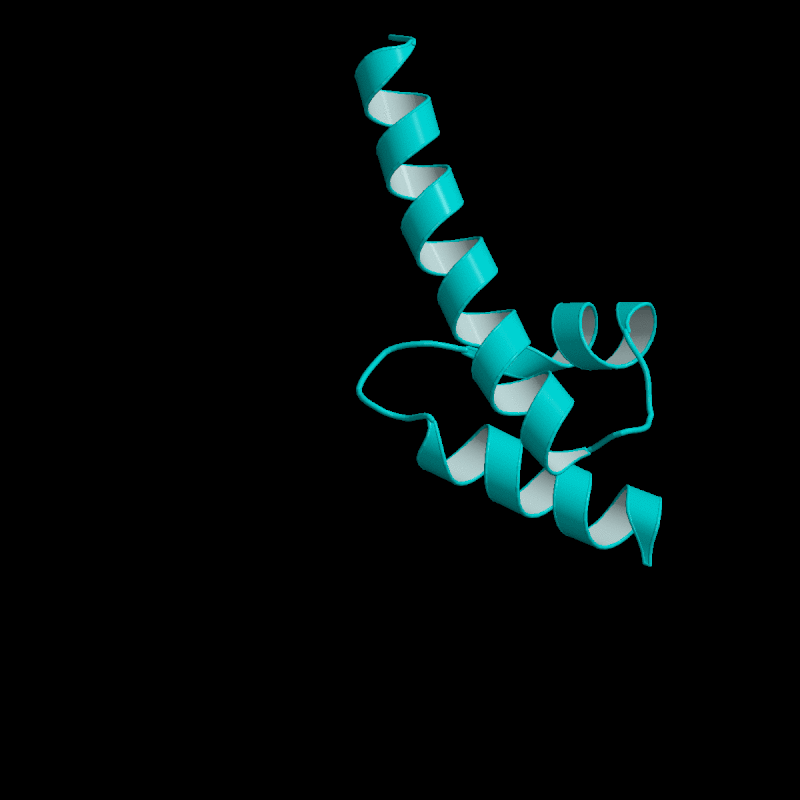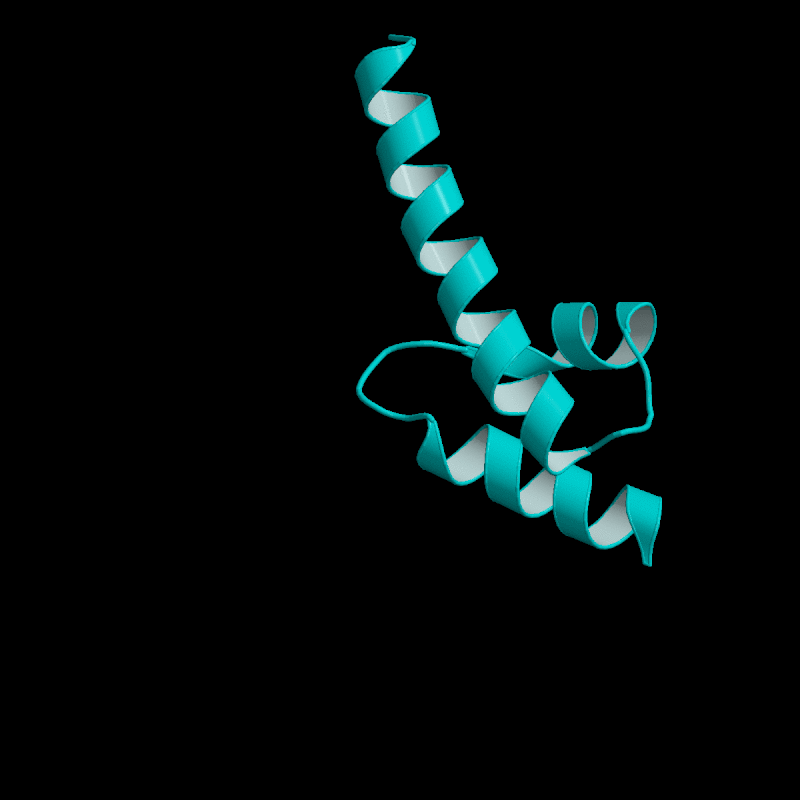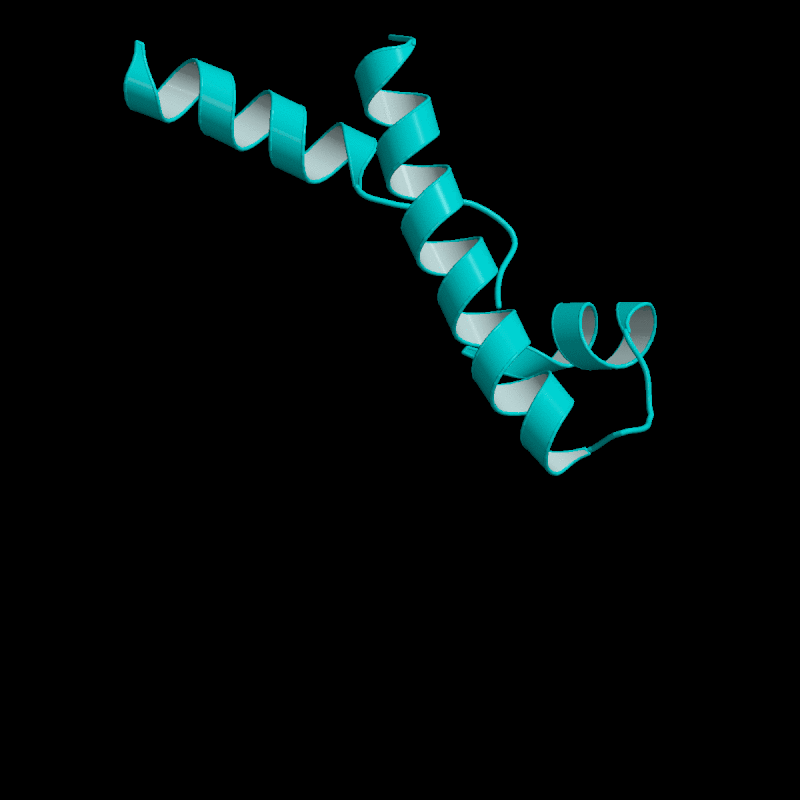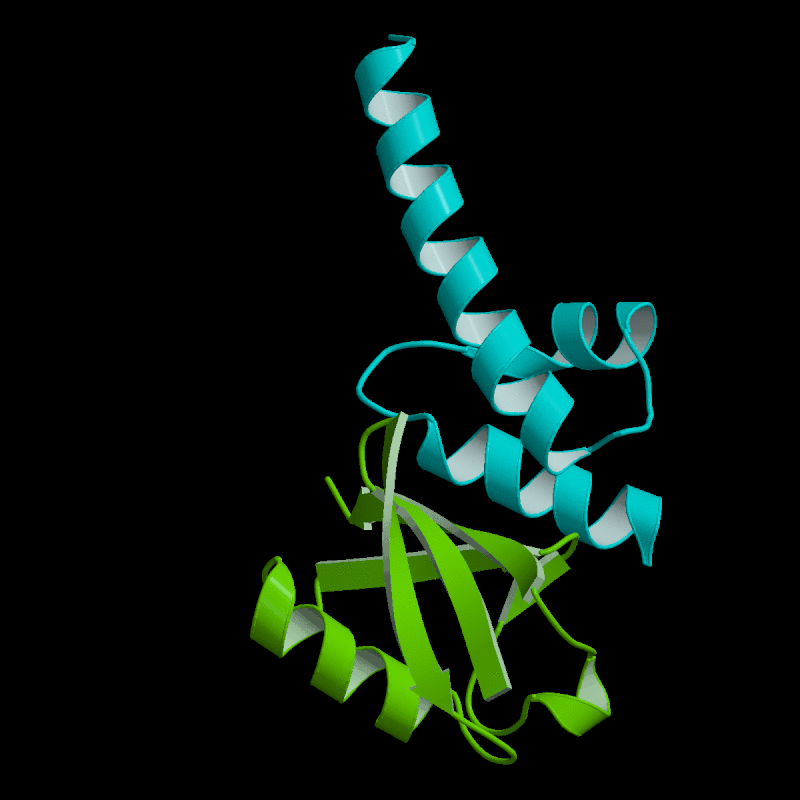 |
Ubiquitylation is a
dynamic regulatory signal that
affects
the activity,
localization and fate determination of
proteins.
The characterized
roles of ubiquitin include acting
as a
sorting signal to
direct protein to degradation in
the
proteasome or in the
lysosome, trafficking in the
endocytic and
biosynthetic pathways,
regulating vesicle and virus
budding
machinery, modifying
histones, regulating
transcriptional
machinery and
controlling intranuclear localization.
Ubiquitylated proteins
are recognized by ubiquitin
binding
domains that transmit
the information conferred by
the new
structure of the
ubiquitylated protein. Our lab is
taking a
structural and
biophysical approaches to
understanding the
molecular mechanism of
ubiquitin recognition. |