


 | |
Experimental
stages
First
meeting
Things to read before the lab:
- Molecular spectroscopy – electronic transitions,
selection rules, transition intensity, Frank-Condon coefficients, relaxation
processes in excited molecules (radiative and non-radiative).
- Electronic levels of aromatic molecules and how
solvent polarity affects
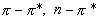 transitions.
transitions.
- Absorption spectra, Beer-Lambert law.
- Fluorescence spectra, usage of reference (QBS),
quantum yield, polarity corrections to quantum yield.
- Polarity scale – ET(30)
Things you would perform:
- Preparation
of solutions
- Measurements
of absorption spectra – absorption peek wavelength and optical density
(OD) at 360 nm.
- Measurement
of fluorescence spectra
At this stage, the students
would be able and required to calculate the quantum yield of the Bimane
molecule.
Second meeting
Things to read before the lab:
Single photon counting technique
– basics and the role of various components in the experimental setup, the
pile-up problem
Things you would
perform:
- Application
of the single photon counting technique to measure the convolution of the
experimental setup with the decay signal of the excited Bimane molecules.
- Calibration
of the time in the experiment and measurement of the bear response of the
experimental setup.
At this stage, the students
would be able and required to find the scaling constant for converting between
A/D channels and real physical time.
Third
Meeting
Things to read before the lab:
Methods of deconvolution
Things you would perform:
Use software to analyze of the
SPC experiments data – deconvolution and extraction of total life times of the
excited Bimane molecules in the various solvents.
Final
report
- Theoretical
background.
- Description
of all experimental methods and problems.
- Analysis
of the results to obtain plots of krad, absorption energy maxima
and fluorescence energy maxima as a function of solvent polarity.
- Discuss
the implication of your results to understanding of molecular conformation
in polar and apolar solutions. Compare
your results to the literature.
|