Steven Dutch, Natural and Applied Sciences, University of Wisconsin - Green
Bay
First-time Visitors: Please visit Site Map and Disclaimer. Use
"Back" to return here.
The spinel minerals have the generic formula XY2O4, where X is a cation with +2 charge and Y is a cation with +3. The most common members include:
| X | Y | Formula | Mineral |
| Mg | Al | MgAl2O4 | Spinel |
| Fe+2 | Fe+3 | Fe+2Fe+3O4=Fe3O4 | Magnetite |
| Fe+2 | Cr | FeCr2O4 | Chromite |
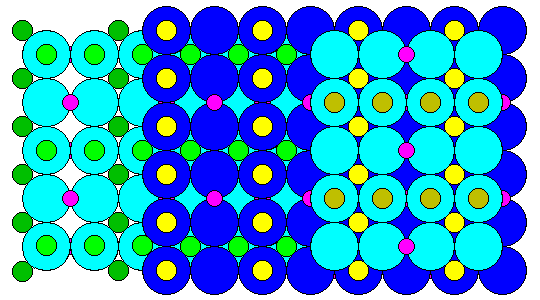
Oxygen atoms in spinel have a cubic close-packed structure. Viewed along a fourfold symmetry axis (perpendicular to a face of a cubic unit cell, spinel looks like this. Three layers of oxygen atoms are shown in alternating shades of blue. Atoms in octahedral sites are shown in shades of green and yellow. Atoms in tetrahedral sites are shown in purple. Contrary to expectations based on ionic radius, in spinel the aluminum atoms are in octahedral sites and the magnesiums in the tetrahedral.

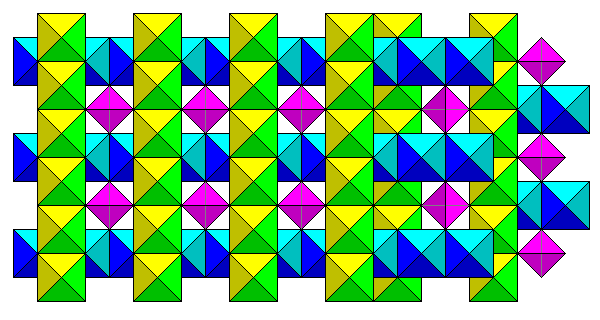
Shown below is a polyhedral representation. Filled octahedra form criss-cross rows, with alternating layers of parallel rows offset as shown on the right side of the diagram. The square holes enclosed by the rows of octahedra are filled with tetrahedra.
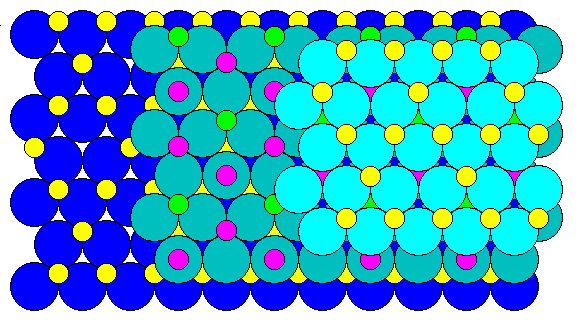
A view perpendicular to the close-packed layers (the octahedral or (111) face) looks like this. Octahedral cations are in yellow and green, tetrahedral in purple. Between oxygen layers we find hexagonal patterns of octahedral cations, and hexagonal patterns of tetrahedral cations with an octahedral cation at the center.
A polyhedral view from the same direction is shown below.
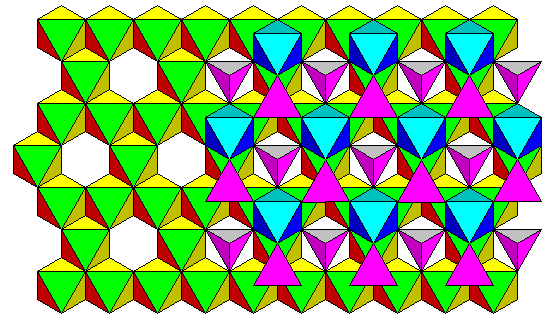
Since there are twice as many filled octahedra as tetrahedra, and the formula for spinel is XY2O4, it is possible to fill all the octahedra with Y (trivalent) atoms and the tetrahedra with X (divalent) atoms. We can't reverse the roles of X and Y and fill all the sites. If we were to try, we could only fill half the octahedra with X, and the tetrahedra would only take up half the Y atoms. The remainder would fill the still-vacant octahedra. Such a structure is called an inverse spinel. Spinel and chromite are normal spinels, magnetite is an inverse spinel. In reality, most spinels are somewhere between the two end states.
Although the spinels are important minerals themselves, their real geological significance is that olivine can also assume a spinel structure under high pressure. The formula of olivine, Mg2SiO4, can be rewritten SiMg2O4, with Si assuming the role of an X cation and Mg the role of a Y cation. This isn't much of a stretch - in an olivine spinel the Mg would be in octahedral coordination and the Si in tetrahedral, which is what they are in olivine anyway. Below is the structure of olivine, showing two layers of octahedra.
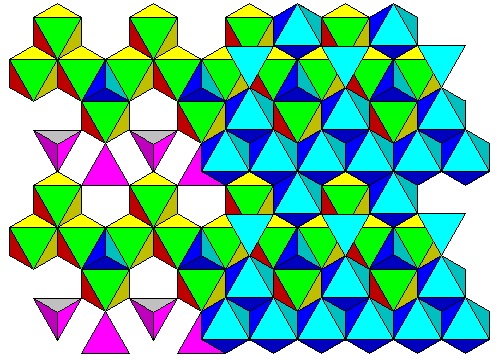
Shown below is the arrangement in spinel. To make the change from olivine to spinel, the continuous rows of octahedra in the top layer have to swap position with the purple terahedra beneath them. The top layer would then look like the top layer in spinel below, but rotated and offset. If we rotate and slide the top layer so the octahedra fit over the tetrahedral voids between octahedra in the bottom layer, and the upward-pointing tetrahedra sit over the rings of octahedra, we have the spinel structure. (This really happens one atom at a time. The geometric description is just a way to allow you to visualize how one structure can evolve into the other. In fact, there is an intermediate form of olivine, beta-olivine, that forms before the final conversion to spinel.)

In both minerals we have close-packed oxygens, silica with tetrahedral coordination and magnesium with octahedral. So how can we gain any space by rearranging the atoms? In olivine the continuous straight rows of octahedra are slightly distorted, probably because of the SiO4 tetrahedra that share octahedral edges. The spinel version of olivine is about 12% more dense. The conversion occurs at around 300-400 km depth in the mantle. The reason we don't find olivine spinels at the surface is probably that the +4 silicon atoms repel each other, and the olivine lattice allows tetrahedra to be a bit farther apart on the whole than the spinel lattice.
The olivine-spinel transition and an analogous transition between pyroxene and perovskite are probably important in driving plate tectonics. High temperature tends to favor low-density polymorphs because the thermally excited atoms take up more room. Thus, when a slab of lithosphere descends, the transition to dense forms occurs at a shallower depth in the cool slab than in the adjacent hotter mantle. Thus the slab becomes heavier than the adjacent mantle and the extra density helps to pull the plate into the subduction zone, a process called slab-pull.
Return to
Mineralogy-Petrology Index
Return to Professor Dutch's Home
Page
Created 22 Sept 1997, Last Update 22 Sept 1997
Not an official UW Green Bay site